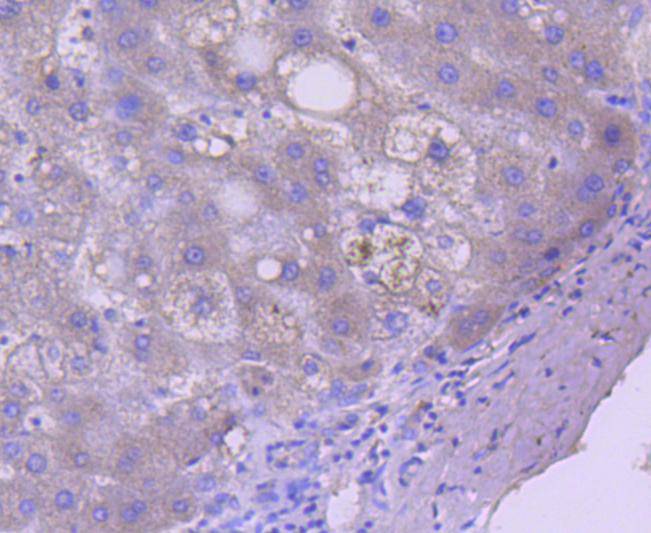
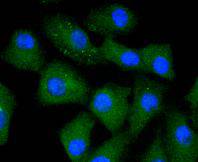
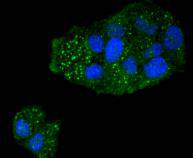
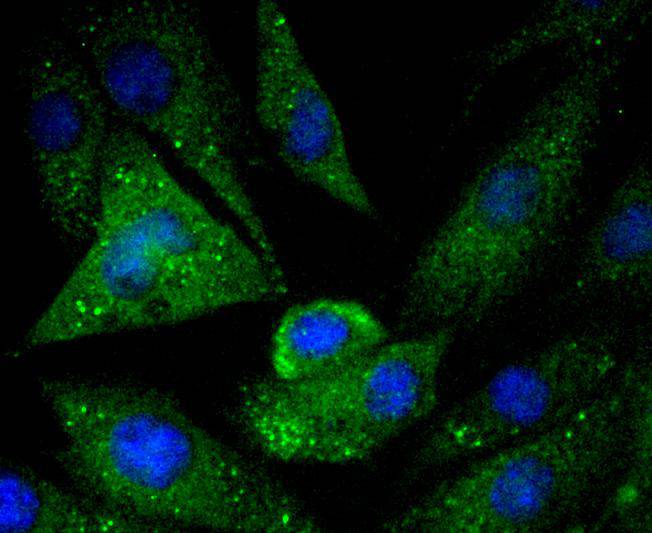
Caspases are cysteine proteases which play important roles in the activation of cytokines and in apoptosis. The ICE subfamily of caspases comprises peptides closely related to caspase-1, which promotes maturation of interleukin 1β (IL-1β) and interleukin-18 (IL-18) by proteolytic cleavage of precursor forms to generate biologically active peptides. Both caspase-4 and caspase-5 are members of the caspase-1 subfamily, and are more closely related to each other than to other homologues. Caspase-5 (also designated ICErel-III, TY, ICH-3 and caspase-12 in mouse), can cleave its own precursor, an activity that requires the cysteine 245 residue. Frameshift mutations in caspase-5 have been identified in MMP tumors of the endometrium, colon, and stomach, indicating the caspase-5 may be a new target gene in the microsatellite mutator pathway for cancer. The human caspase 5 gene maps to chromosome 11q22.2-q22.3 and encodes a protein whose expression is barely detectable in most tissues except brain, with highest expression levels being found in lung, liver and skeletal muscle.