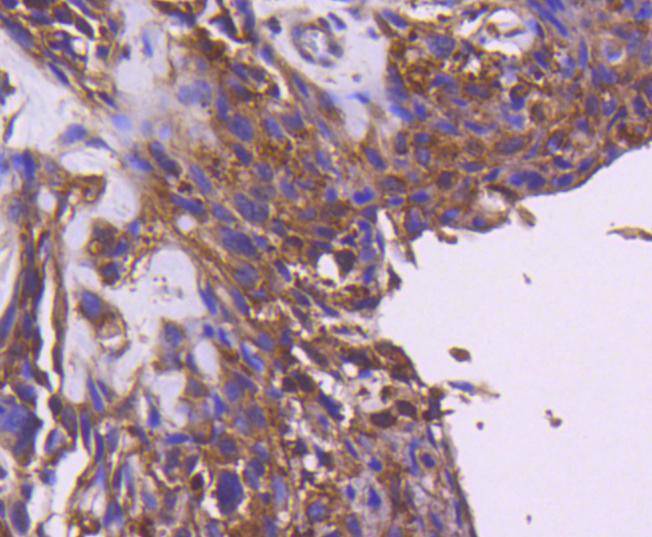
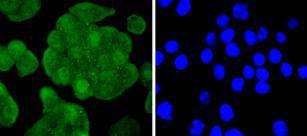
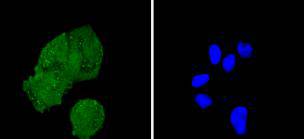
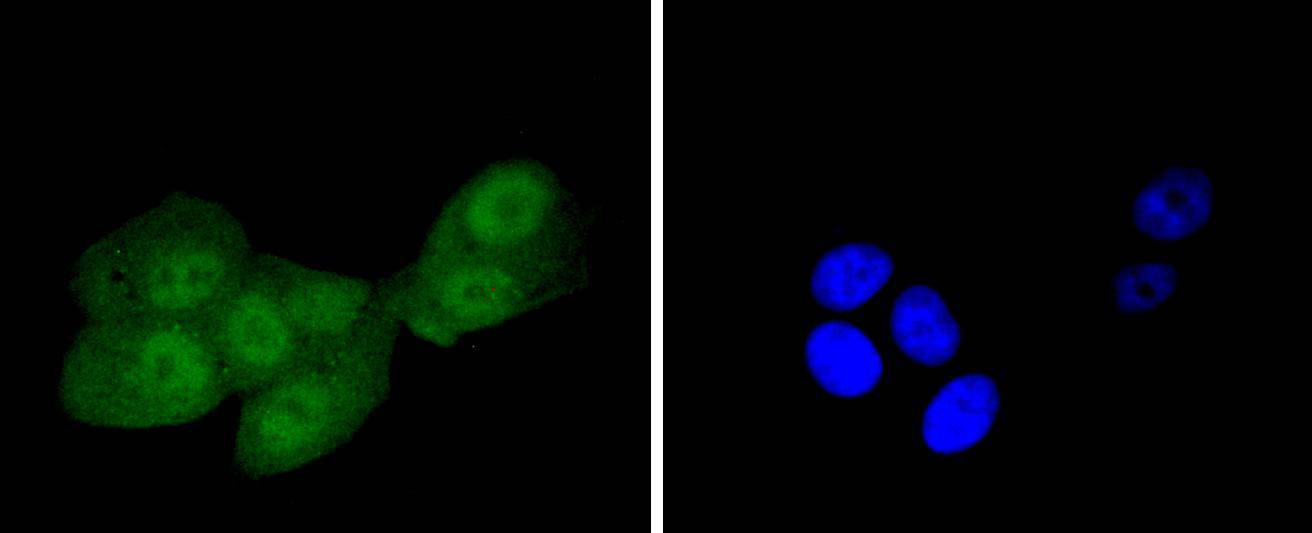
The insulin receptor substrate-1 (IRS-1), a protein major substrate of the insulin receptor, is phosphorylated in response to stimulation of cells by insulin, insulin-like growth factor 1 (IGF-1) and interleukin 4 (IL-4). IRS-1 is phosphorylated on serine, threonine and tyrosine residues in a variety of tissues. An insulin-sensitive serine/threonine kinase casein kinase II mediates a portion of the insulin-stimulated serine/threonine phosphorylation of overexpressed IRS-1 in vivo. Thr 502 is identified as the major casein kinase II-catalyzed phosphorylation site in rat IRS-1, and Ser 99 is an additional phosphorylation site catalyzed by casein kinase II. Thus, casein kinase II-catalyzed phosphorylation of IRS-1 may be a component of the intracellular insulin signaling cascade. IRS-1 contains three putative binding sites for 14-3-3 (Ser 270, Ser 374 and Ser 641) and the motif around Ser 270 is located in the phosphotyrosine binding domain of IRS-1, which is responsible for the interaction with the insulin receptor. The association of 14-3-3 with IRS-1 increases significantly upon treatment with okadaic acid, a potent serine/ threonine phosphatase inhibitor. Therefore, the association of 14-3-3 protein may play a role in the regulation of insulin sensitivity by interrupting the association between the insulin receptor and IRS-1.