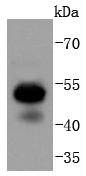
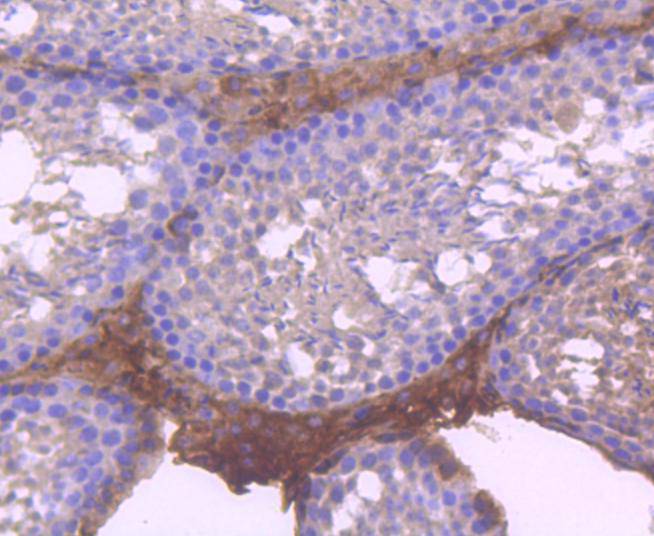
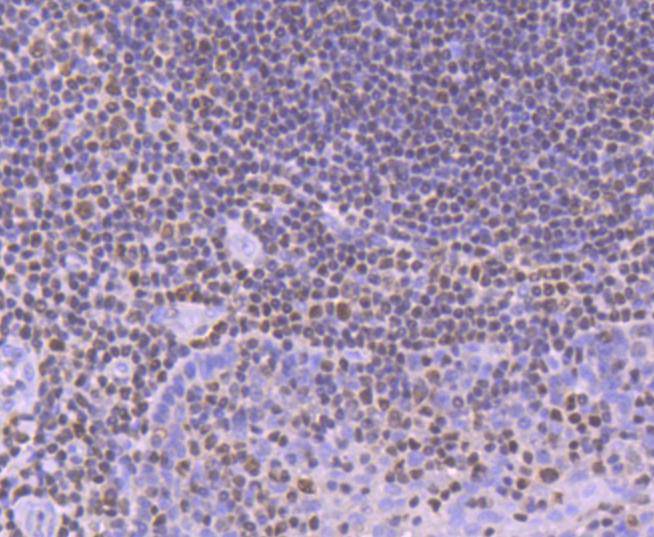
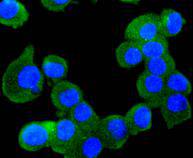
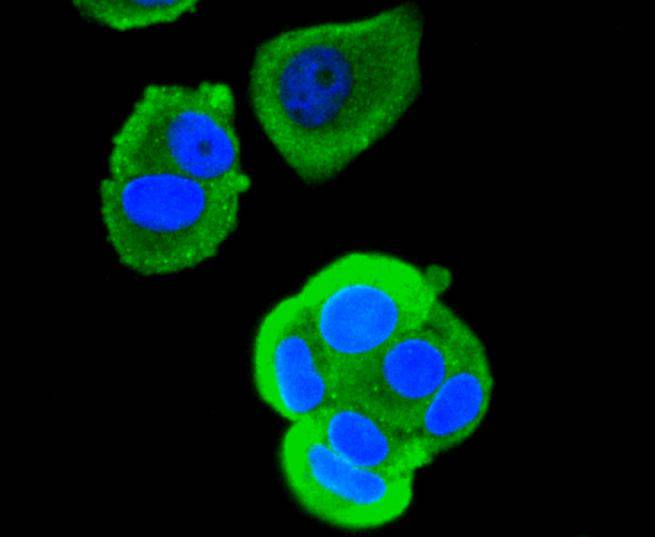
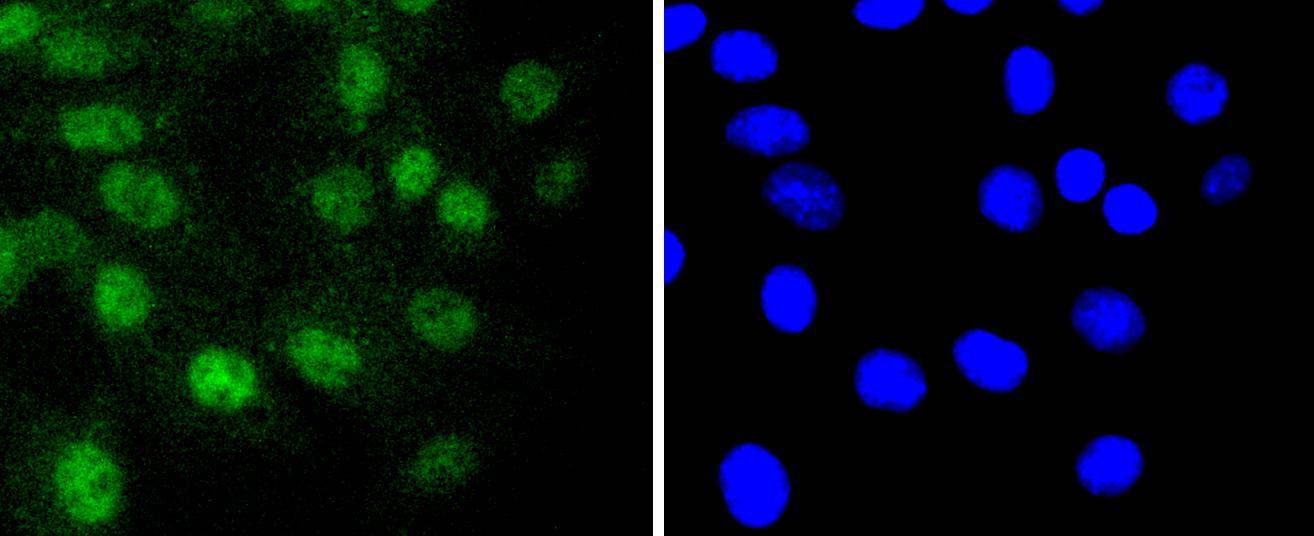
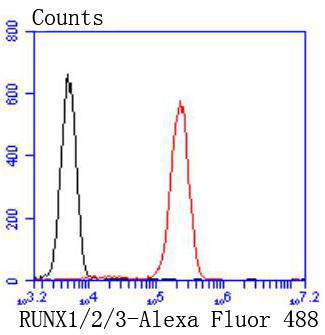
The mammalian Runt-related transcription factor (RUNX) family comprises three members, RUNX1 (also designated AML-1, PEBP2αB, CBFA2), RUNX2 (also designated AML-3, PEBP2αA, CBFA1, Osf2) and RUNX3 (also designated AML-2, PEBPαC, CBFA3). RUNX family members are DNA-binding proteins that regulate the expression of genes involved in cellular differentiation and cell cycle progression. RUNX1 is involved in hematopoiesis and is frequently targeted in human leukemia by chromosomal translocations that fuse the DNA-binding domain of RUNX1 to other transcription factors and corepressor molecules. In addition to its role in leukemogenesis, RUNX1 is also involved in sensory neuron diversification. RUNX1 promotes axonal growth, is selectively expressed in neural crest-derived TrkA+ sensory neurons and mediates TrkA transactivation in migratory neural crest cells. RUNX2 is essential for skeletal mineralization in that it stimulates osteoblast differentiation of mesenchymal stem cells, promotes chondrocyte hypertrophy and contributes to endothelial cell migration and vascular invasion of developing bones. Regulating RUNX2 expression may be a useful therapeutic tool for promoting bone formation. Mutations in the C-terminus of RUNX2 are associated with cleidocranial dysplasia syndrome, an autosomal-dominant skeletal dysplasia syndrome that is characterized by widely patent calvarial sutures, clavicular hypoplasia, supernumerary teeth, and short stature. RUNX3 is expressed in cells of hematopoietic origin, including myeloid and B-cell lines and spleen. By playing a role in controlling the growth and differentiation of gastric epithelial cells, RUNX3 is a strong candidate as a gastric cancer tumor suppressor. Specifically, hypermethylation inactivates the gene encoding RUNX3. The detection of hypermethylation at multiple regions within the RUNX3 CpG island may aid in the diagnosis and risk assessment of gastric cancer.