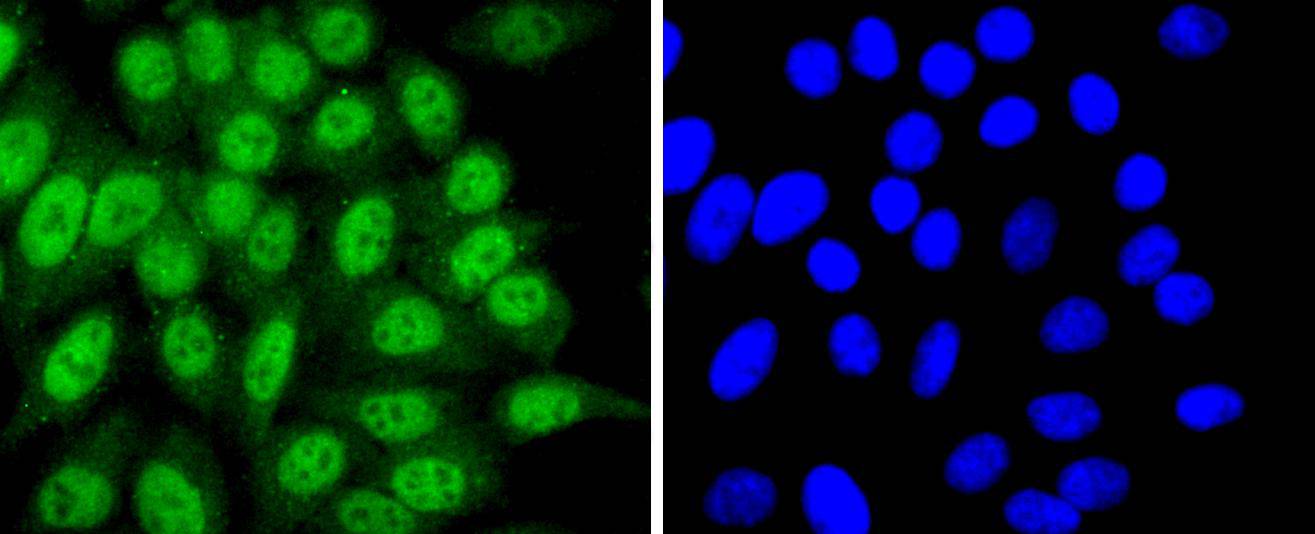
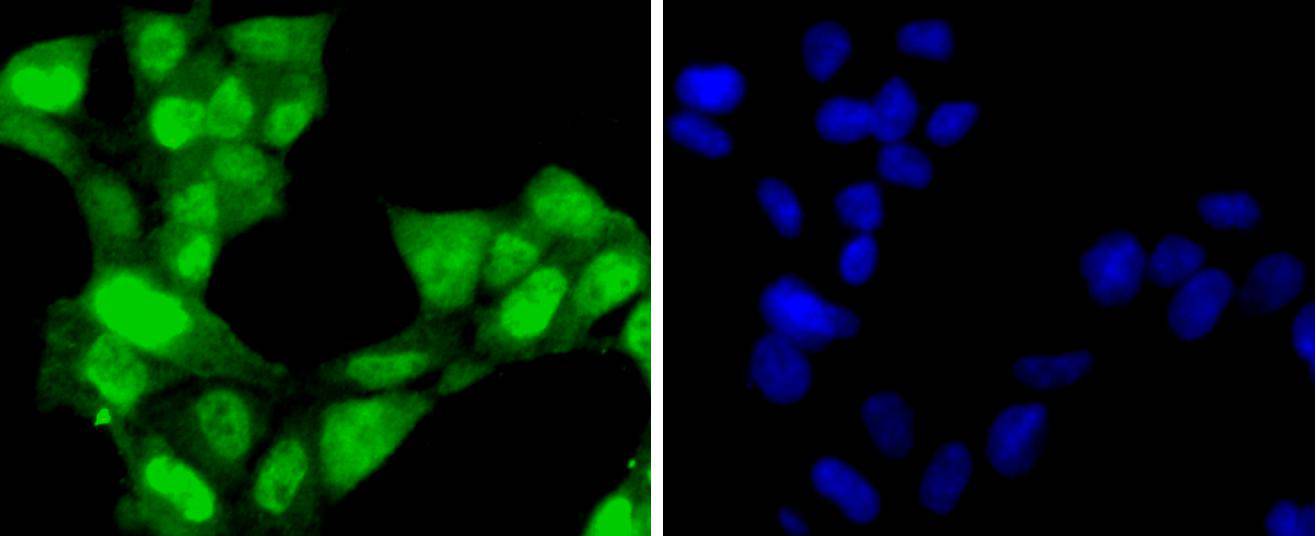
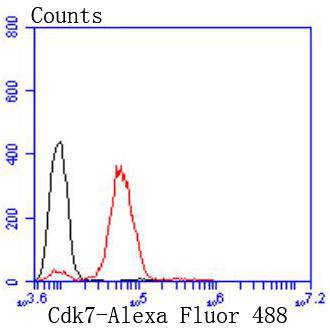
Progression through the cell cycle requires activation of a series of enzymes designated cyclin dependent kinases (Cdks). The monomeric catalytic subunit Cdk2, a critical enzyme for initiation of cell cycle progression, is completely inactive. Partial activation is achieved by the binding of regulatory cyclins such as cyclin D1, while full activation requires additional phosphorylation at Thr 160. The enzyme responsible for the phosphorylation of Cdk2 on Thr 160 and also of Cdc2 p34 on Thr 161, designated Cdk-activating kinase (CAK), has been partially purified and shown to be comprised of a catalytic subunit and a regulatory subunit. The catalytic subunit, designated Cdk7, has been identified as the mammalian homolog of MO15, a protein kinase demonstrated in starfish and Xenopus. The regulatory subunit is a novel cyclin (cyclin H) and is required for activation of Cdk7. Like other Cdks, Cdk7 contains a conserved threonine residue required for full activity; mutation of this residue severely reduces CAK activity.