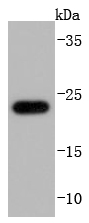
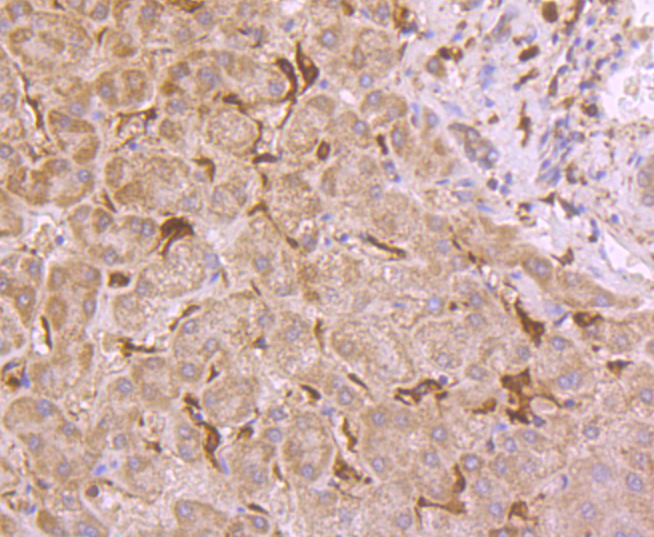
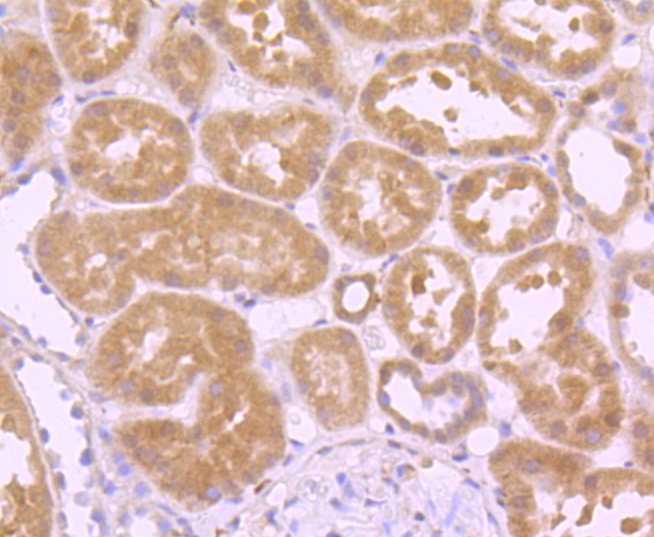
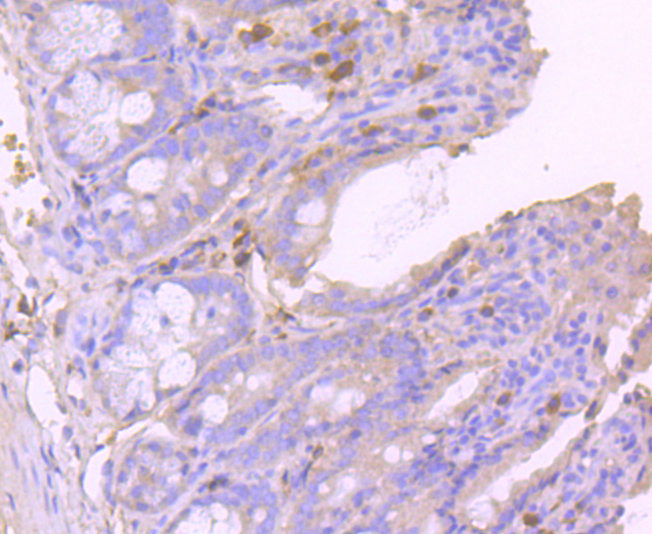
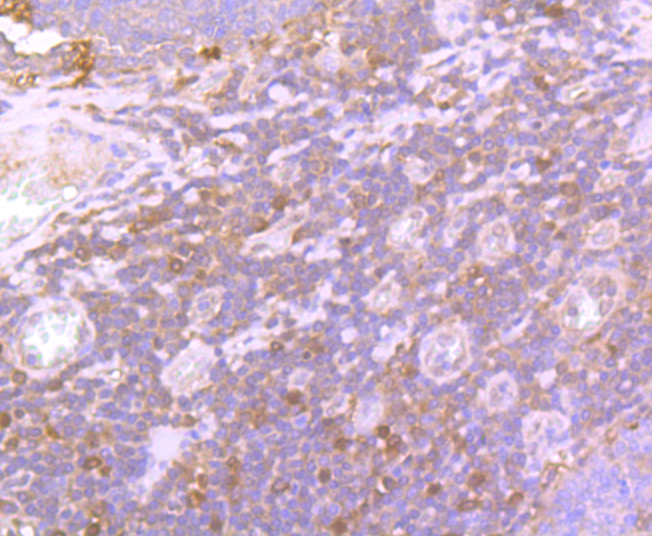
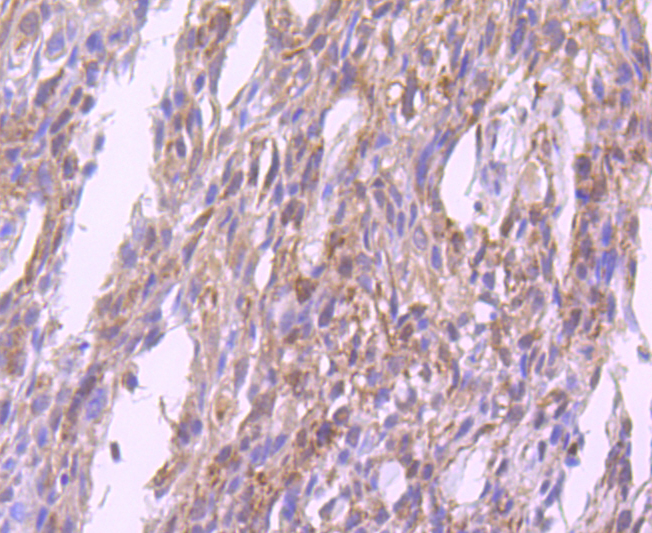
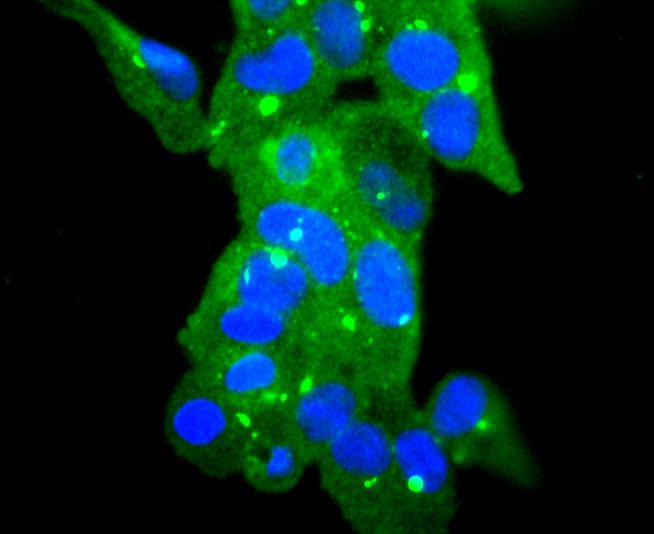
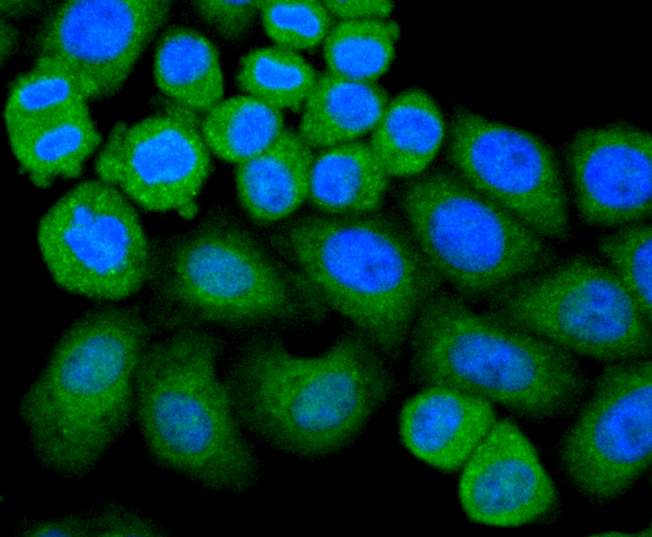
Glutathione peroxidase (GPx) enzymes are generally selenium-containing tetrameric glycoproteins that help prevent lipid peroxidation of cell membranes. GPx enzymes reduce lipid hydroperoxides to alcohols, and reduce free hydrogen peroxide to water. GPx members are among the few proteins known in higher vertebrates to contain selenocysteine, which occurs at the active site of glutathione peroxidase and is coded by the nonsense (stop) codon TGA. There are eight GPx homologs (GPx-1-8). GPx-1, Gpx-2 and Gpx-3 exist as homotetramers. Gpx-4 has a high tendancy to form high molecular weight oligomers. GPx-1 plays an important role in the antioxidant defense of the vascular wall and neural cells in response to oxidative stress. GPx-2 is the major isoform in the lungs and its basal or inducible expression is dependent on Nrf2. GPx-3 is under regulation by hypoxic stress and the expression and deficiency of GPx-3 is associated with cardiovascular disease and stroke. GPx-5 is selenium-independent; it is bound to the acrosome of sperm, where it may protect sperm from premature acrosome reaction in the epididymis.