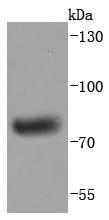
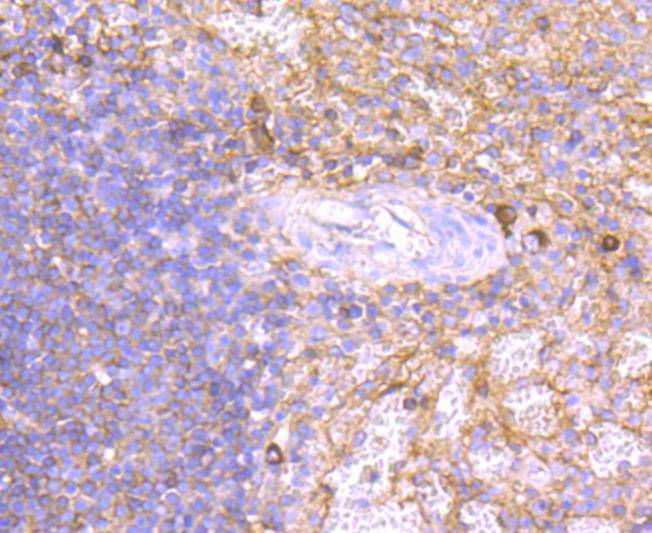
Immunoglobulin M (IgM) is the largest circulating antibody molecule in humans. It consists of a heavy chain (?-chain) and a light chain (κ- or λ-chain), as well as 5 base units and 10 binding sites, though it cannot bind all 10 simultaneously because of steric hindrance. IgM chain C refers to the constant region of the IgM heavy chain that is involved in immune regulation. IgM forms polymers by covalently linking multiple immunoglobulins together with disulfide bonds. It normally exists as a pentamer, but occasionally as a hexamer. Because of its polymeric nature, IgM has high avidity, and it is especially effective at complement activation. Due to its large size, IgM does not diffuse well, and it is found in the interstitium in very low amounts. IgM is mainly found in serum; however, because of the J chain, it is also important as a secretory immunoglobulin. IgM is the first immunoglobulin expressed by mature B cells, and it normally appears early in the course of an infection and does not reappear after further exposure.