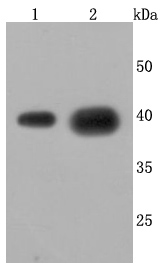
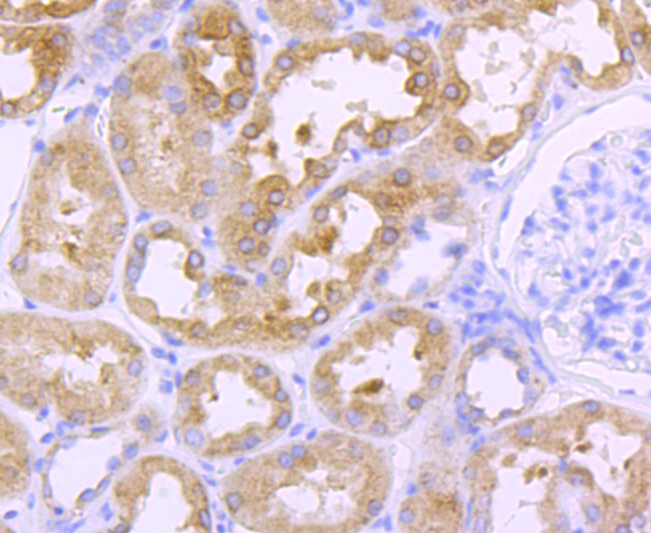
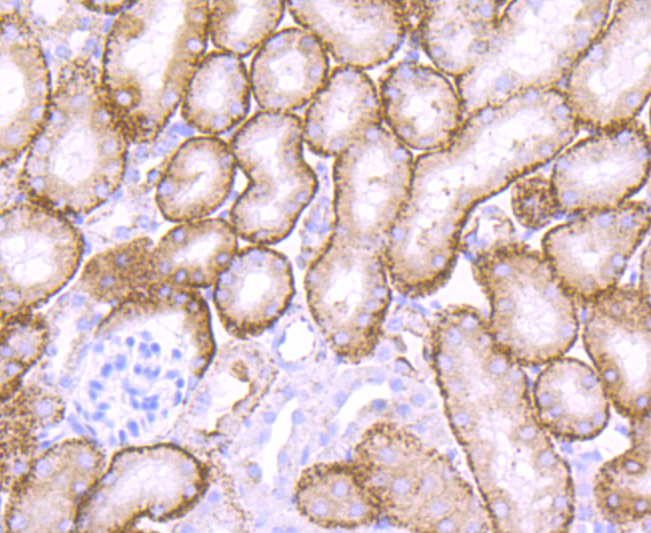
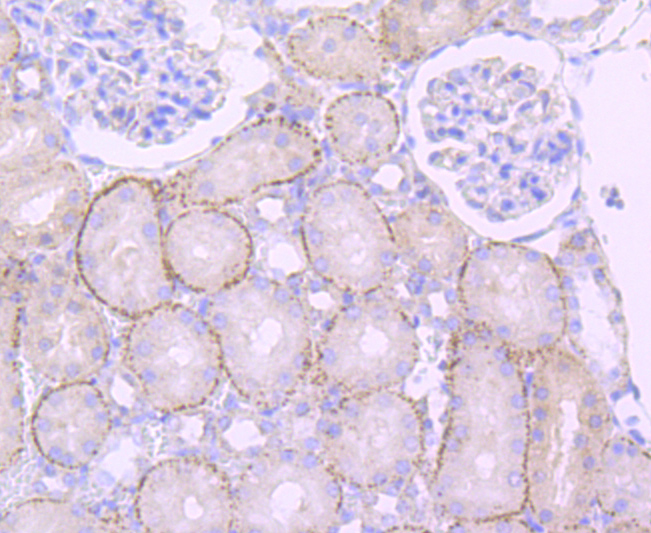
The cathepsin family of proteolytic enzymes contains several diverse classes of proteases. The cysteine protease class comprises cathepsins B, L, H, K, S, and O. The aspartyl protease class is composed of cathepsins D and E. Cathepsin G is in the serine protease class. Most cathepsins are lysosomal and each is involved in cellular metabolism, participating in various events such as peptide biosynthesis and protein degradation. Cathepsin L (also designated major excreted protein, MEP or CATL) is a member of the peptidase C1 family and has been identified as a protein that is most closely related to cathepsin H. It is a lysosomal cysteine proteinase that mediates intracellular protein catabolism for collagen, elastin and ?-1 protease inhibitor. Cathepsin L is a dimer composed of disulfide-linked heavy and light chains, both produced from a single protein precursor. At least two transcript variants encoding the same protein have been found for this gene. Transformed mouse fibroblasts stimulated by growth factors or tumor promoters secrete a form of cathepsin L.