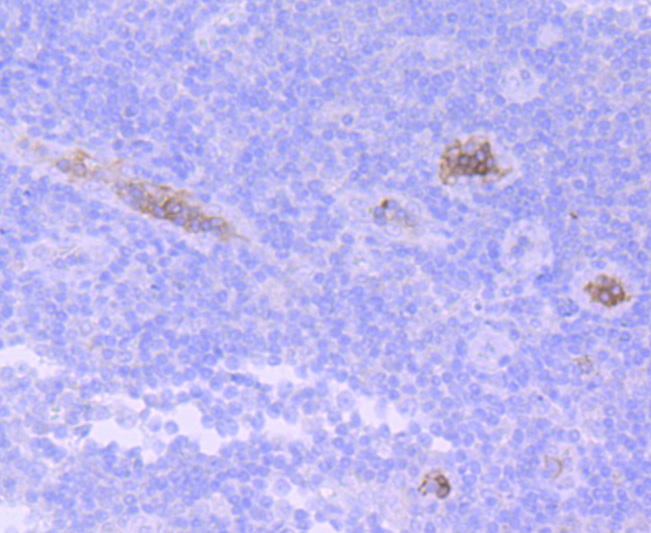
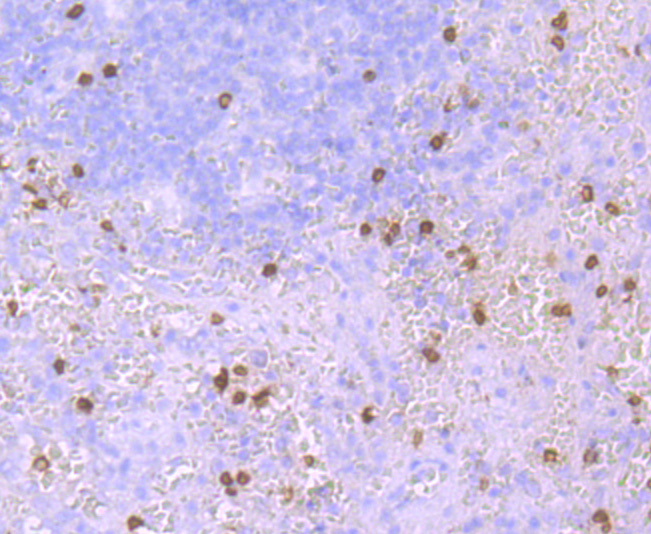
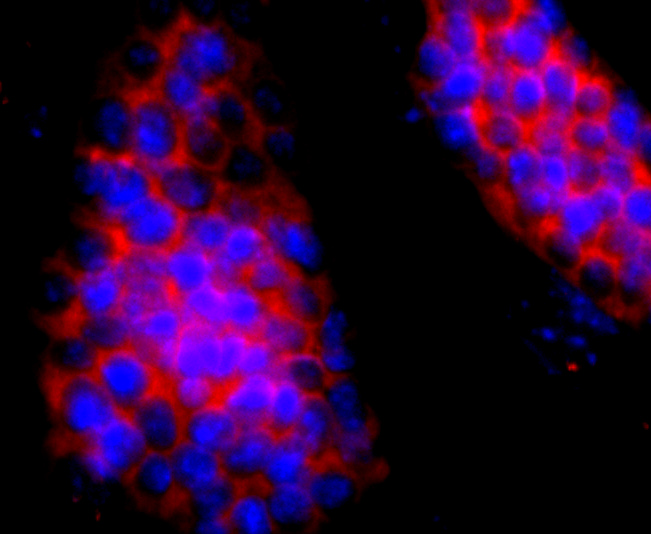
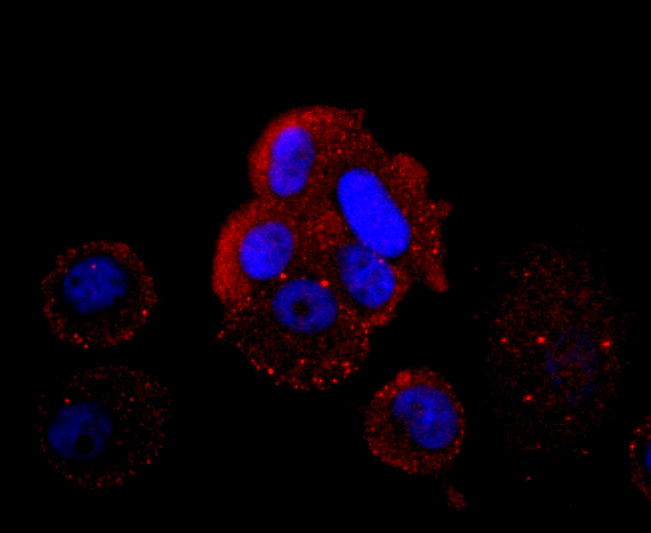
Hemoglobin (Hgb) is coupled to four iron-binding, methene-linked tetrapyrrole rings (heme). The α (16p13.3; 5-ζ-pseudoz-pseudo α2-pseudo α1-α2-α1-?1-3) and β (11p15.5) globin loci determine the basic hemoglobin structure. The globin portion of hemoglobin consists of two α chains and two β chains arranged in pairs forming a tetramer. Each of the four globin chains covalently associates with a heme group. The bonds between α and β chains are weaker than between similar globin chains, thereby forming a cleavage plane that is important for oxygen binding and release. High affinity for oxygen occurs upon relaxation of the α1-β2 cleavage plane. When the two α1-β2 interfaces are closely bound, hemoglobin has a low affinity for oxygen. Hb A, which contains two α chains plus two β chains, comprises 97% of total circulating hemoglobin. The remaining 3% of total circulating hemoglobin is comprised of Hb A-2, which consists of two α chains plus two δ chains, and fetal hemoglobin (Hb F), which consists of two α chains together with two γ chains.