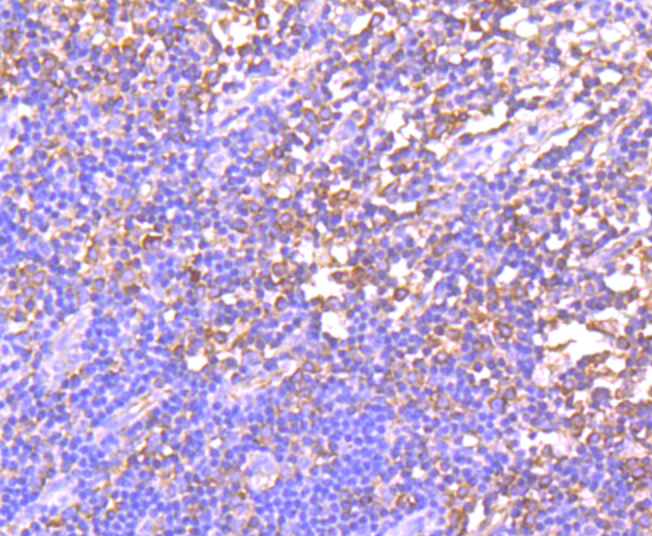
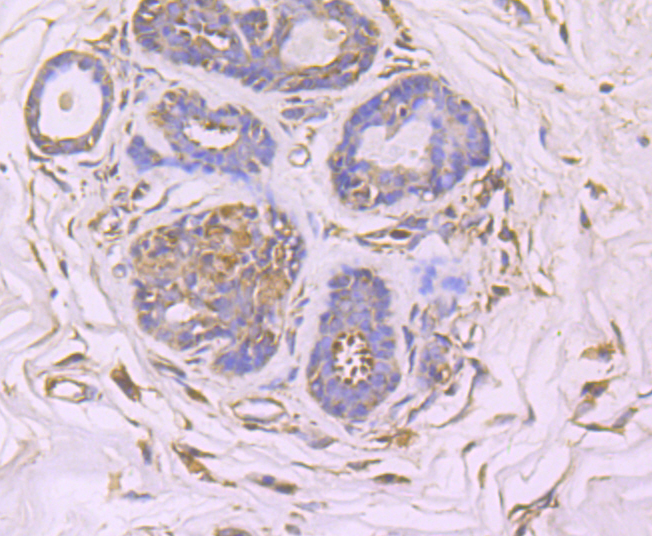
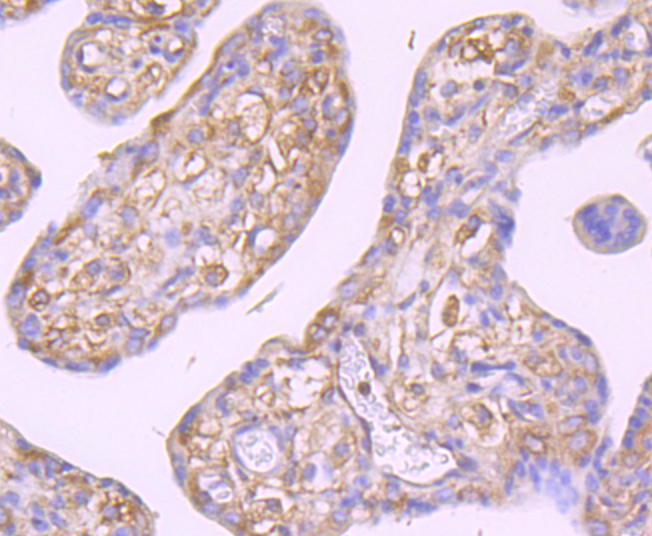
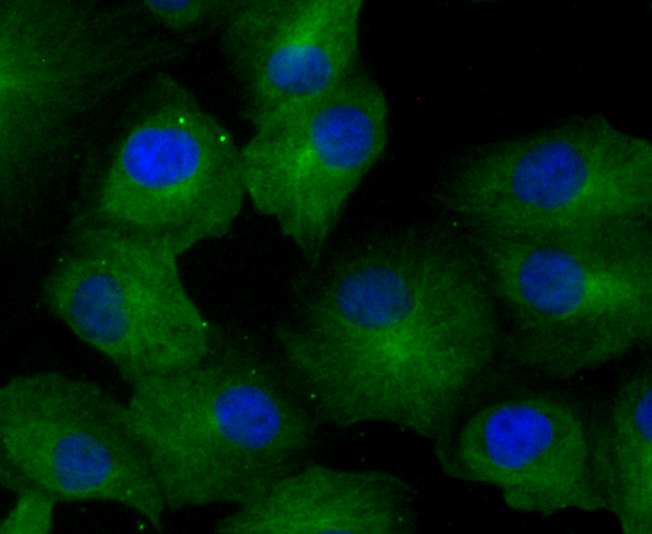
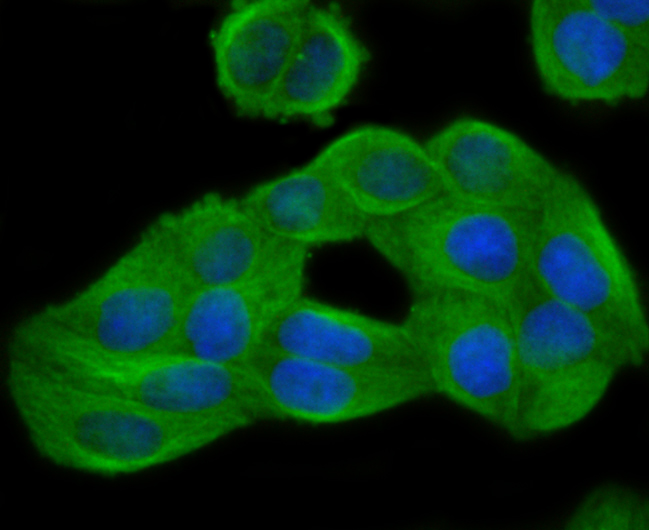
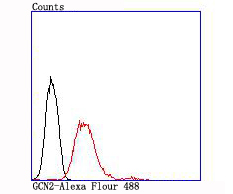
The family of stress-responsive protein kinases include HRI (heme-regulated inhibitor or EIF2AK1), PKR (EIF2AK2 or TIK), PERK (EIF2AK3) and GCN2 (EIF2AK4). These proteins phosphorylate the eukaryotic translation initiation factor 2α (eIF2α) on Ser 51 to regulate general and gene-specific protein synthesis. Phosphorylated eIF2α acts as an inhibitor of its guanine nucleotide exchange factor eIF2B. GCN2, a unique eIF2α kinase, exists in all eukaryotes from yeast to mammals. In mammals, expression of GCN2 is highest in liver and brain tissues. GCN2 primarily initiates the phosphorylation of eIF2α in response to UV, but has been shown to increase phosphorylation activity in response to serum starvation. Also, substitution of Asp 83 for Ala on eIF2α results in impaired phosphorylation by GCN2 and PKR, suggesting a contribution of remote residues to kinase-substrate recognition.