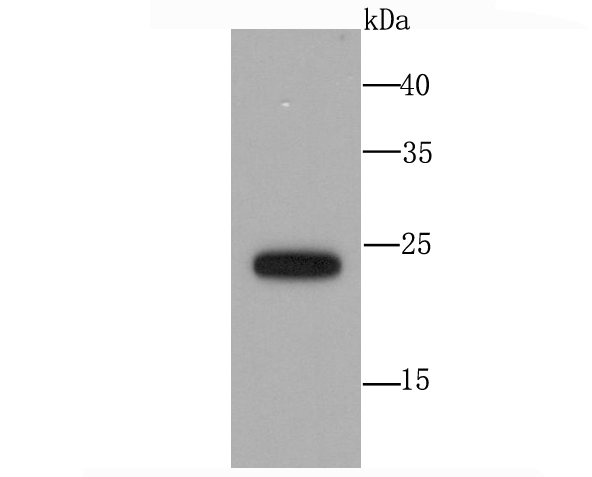
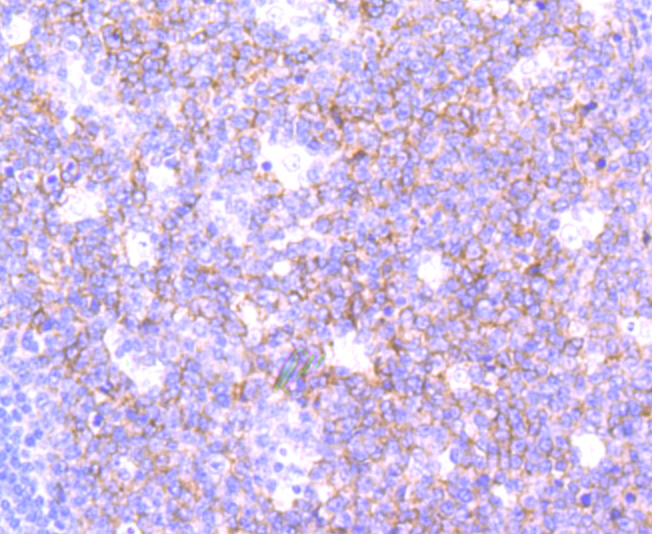
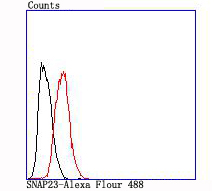
In eukaryotic cells, the Golgi apparatus receives newly synthesized proteins from the endoplasmic reticulum and delivers them after covalent modification to their destination in the cell. For membrane-directed proteins this process is believed to be carried out via vesicular transport. Correct vesicular transport is determined by specific pairing of vesicle-associated SNAREs (v-SNAREs) with those on the target membrane (t-SNAREs). This complex then recruits soluble NSF attachment proteins (SNAPs) and N-ethylmaleimide-sensitive factor (NSF) to form the highly stable SNAP receptor (SNARE) complex. The formation of a SNARE complex pulls the vesicle and target membrane together and may provide the energy to drive fusion of the lipid bilayers. A SNAP 25 related t-SNARE protein, SNAP 23, is required for exocytosis, suggesting that SNAP 23 may play an important role in membrane fusion events. The human SNAP 23 gene encodes two SNAP 23 isoforms, SNAP 23A and SNAP 23B. SNAP 23B is identical to a fragment of SNAP 23A, but SNAP 23B lacks 53 amino acid residues (90 to 142) that are present in SNAP 23A. SNAP 23 is ubiquitously expressed and is an important regulator of transport vesicle docking and fusion in all mammalian cells.