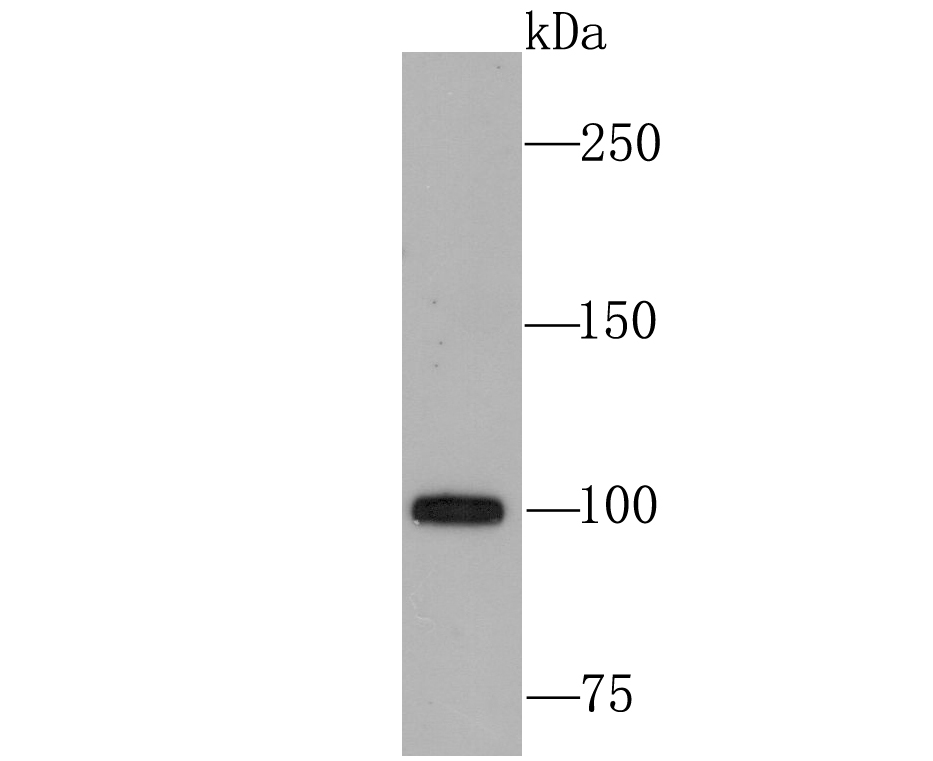
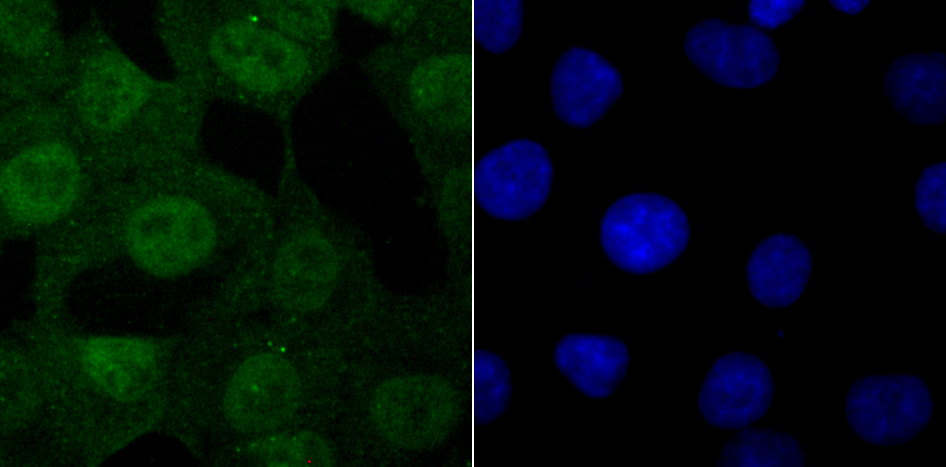
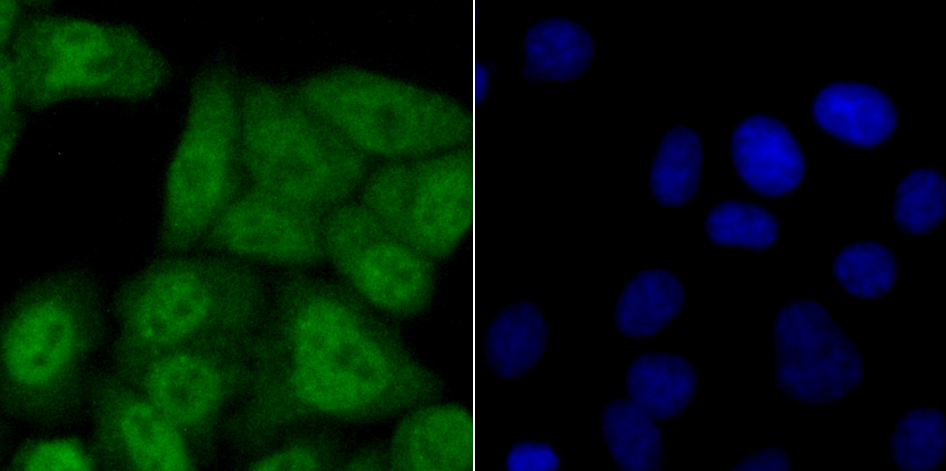
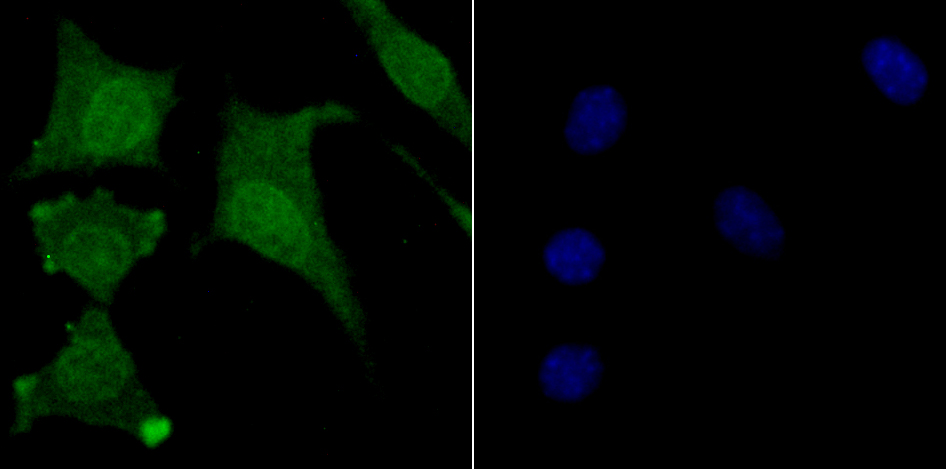
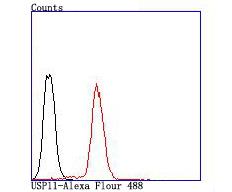
The ubiquitin (Ub) pathway involves three sequential enzymatic steps that facilitate the conjugation of Ub and Ub-like molecules to specific protein substrates. Through the use of a wide range of enzymes that can add or remove ubiquitin, the Ub pathway controls many intracellular processes such as signal transduction, transcriptional activation and cell cycle progression. USP11 (ubiquitin specific peptidase 11), also known as UHX1, is a 920 amino acid deubiquitinating enzyme that participates in the Ub pathway. Localized to the nucleus, USP11 associates with both Ran BP-M (Ran binding protein M) and with the tumor suppressor BRCA2. Through these associations, USP11 functions to either inhibit ubiquitination of these proteins or to remove ubiquitin residues that have already been attached to these proteins. USP11 is implicated in several X-linked retinal diseases and, due to its ability to deubiquitinate BRCA2, may play a role in tumor suppression.