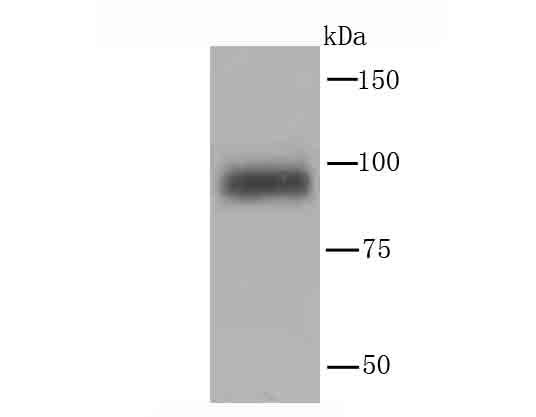
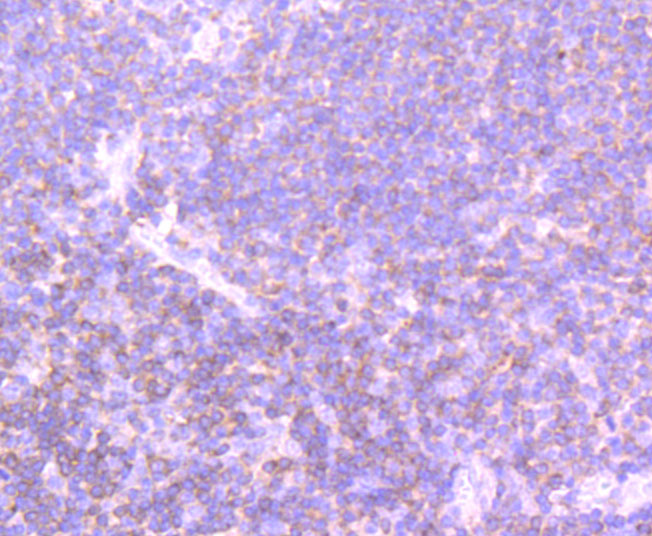
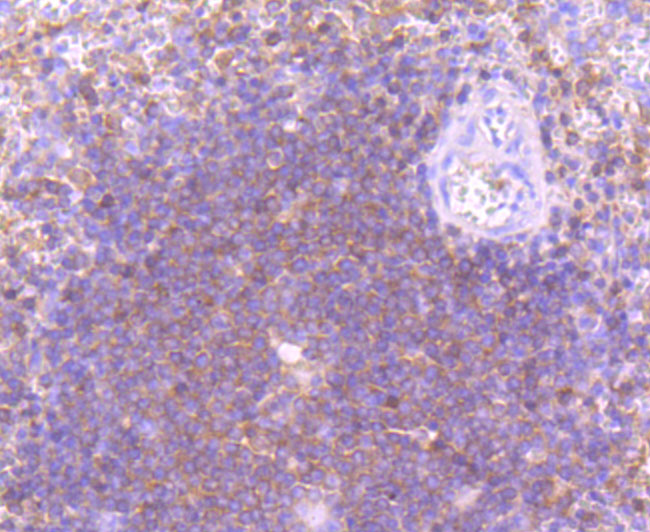
Phosphatidylinositol 3-kinase (PI 3-kinase) is composed of p85 and p110 subunits. p85 lacks PI 3-kinase activity and acts as an adapter, coupling p110 to activated protein tyrosine kinase. Two forms of p85 have been described (p85α and p85β), each possessing one SH3 and two SH2 domains. Various p110 forms have been identified. p110α and p110β interact with p85α, and p110α has also been shown to interact with p85β in vitro. It has been shown to bind p85α and β, but it apparently does not phosphorylate these subunits. p110δ has the capacity to autophosphorylate and results in the nearly complete inactivation of the lipid kinase activity. Interestingly, p110γ does not interact with the p85 subunits and has been shown to be activated by α and βγ heterotrimeric G proteins. Two p110δ isoforms have been identified and are widely expressed. Isoform 1 is expressed predominantly in leukocytes while isoform 2 is expressed in normal thymus, lung and spleen tissues.