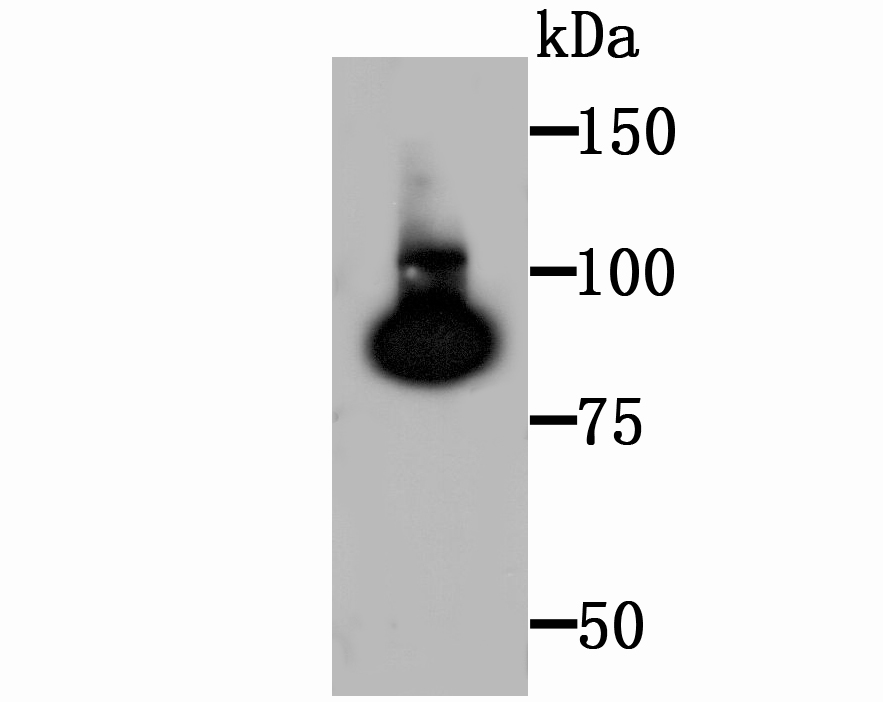
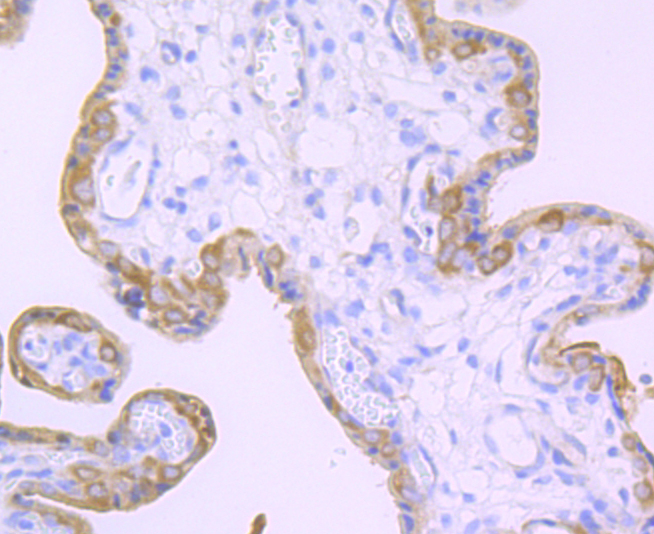
Terminally differentiating mammalian epidermal cells acquire an insoluble, 10 to 20 nm thick protein deposit on the intracellular surface of the plasma membrane known as the cross-linked cell envelope (CE). The CE is a component of the epidermis that is generated through formation of disulfide bonds and g-glutamyl-lysine isodipeptide bonds, which are formed by the action of transglutaminases (TGases). TGases are intercellularly localizing, Ca2+-dependent enzymes that catalyze the formation of isopeptide bonds by transferring an amine on to glutaminyl residues, thereby cross-linking glutamine residues and lysine residues in substrate proteins. TGases influence numerous biological processes, including blood coagulation, epidermal differentiation, seminal fluid coagulation, fertilization, cell differentiation and apoptosis. Human keratinocyte transglutaminase (TGase1) is a membrane associated, 817 amino acid protein. Human tissue transglutaminase (TGase2) is an endothelial cell specific, 687 amino acid protein.