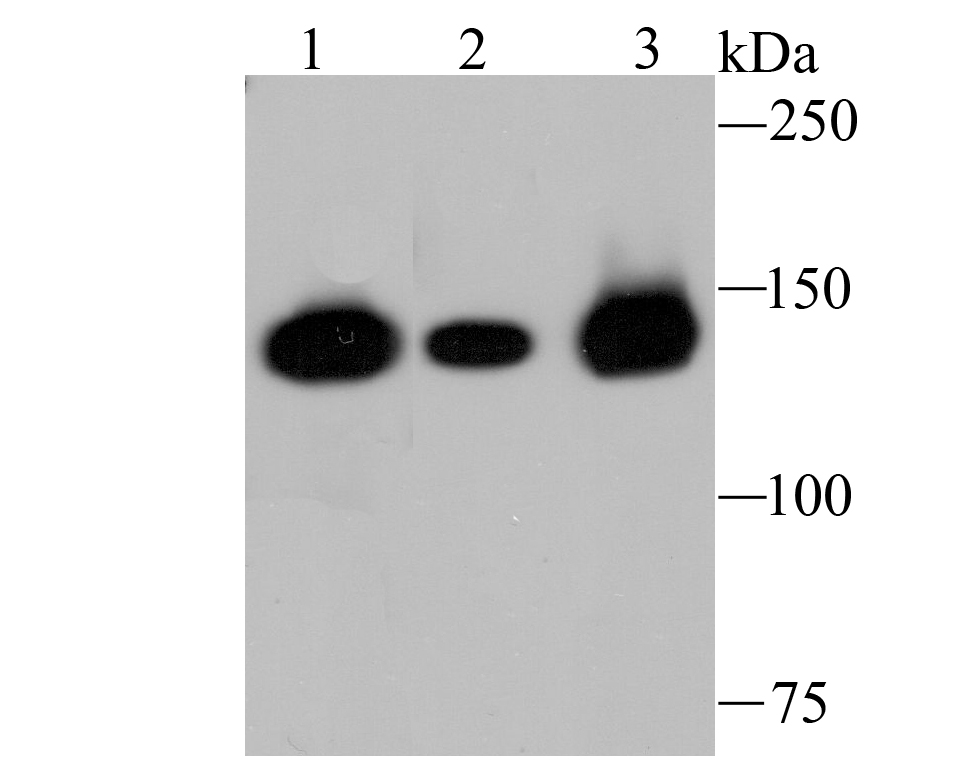
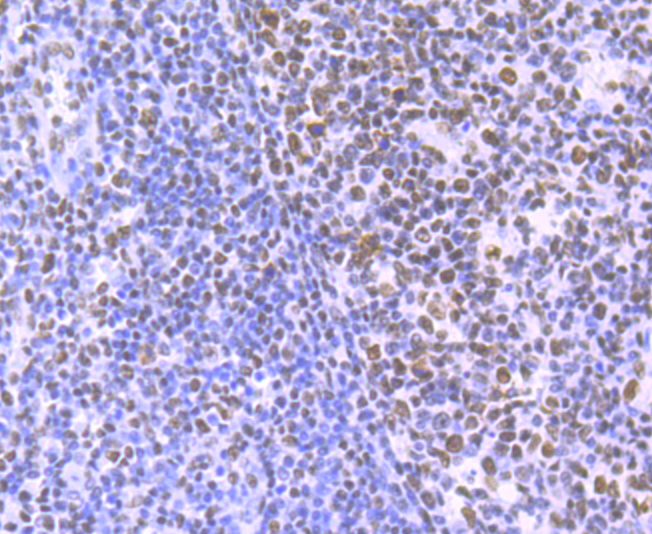
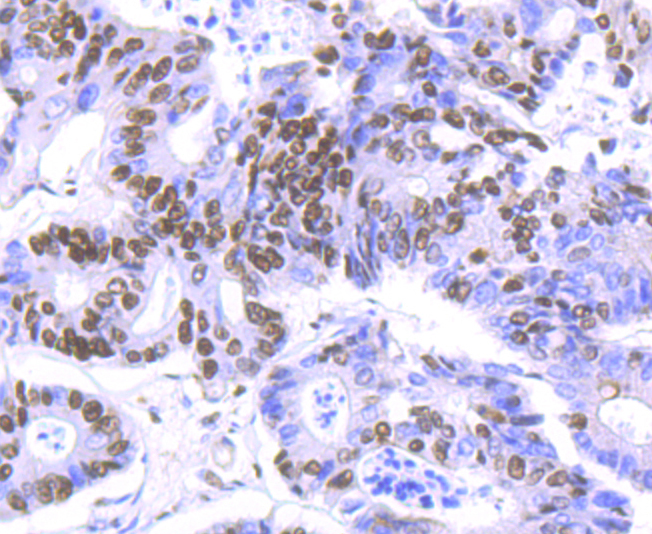
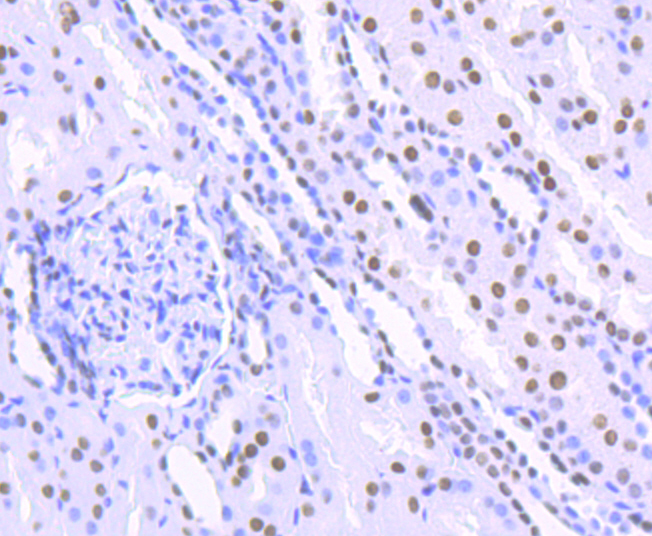
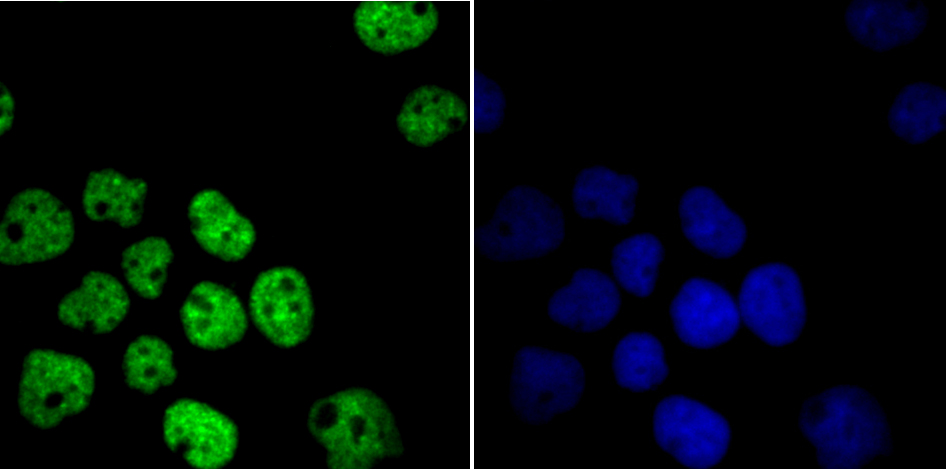
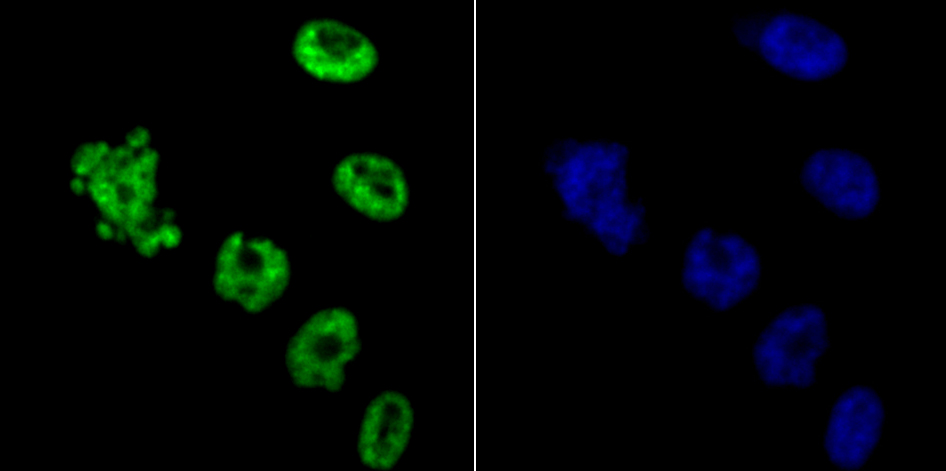
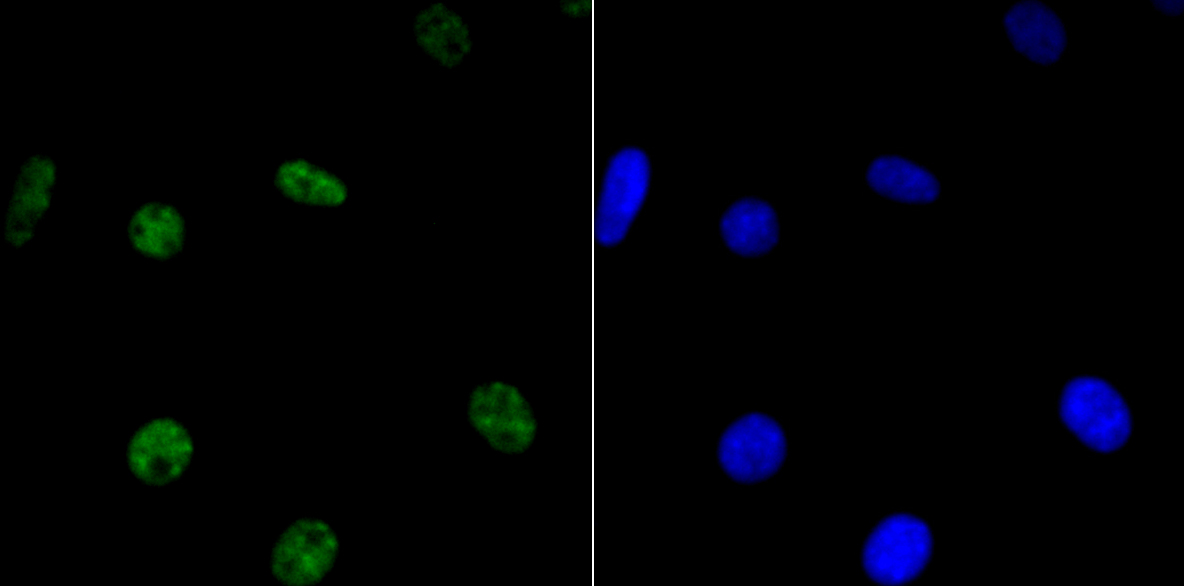
In the intact cell, DNA closely associates with histones and other nuclear proteins to form chromatin. The remodeling of chromatin is a critical component of transcriptional regulation and the acetylation of nucleosomal histones is a major source of this remodeling. Acetylation of lysine residues in the amino terminal tail domain of histone results in an allosteric change in the nucleosomal conformation and an increased accessibility to transcription factors by DNA. Several mammalian proteins function as nuclear histone acetylases, including GCN5, PCAF (p300/CBP-associated factor), p300/CBP, HAT1 and the TFIID subunit TAF II p250. Conversely, the deacetylation of histones is associated with transcriptional silencing. The histone deacetylases (HDAC) include HDAC1-9. HDAC9 and HDAC9a are two alternatively spliced isoforms of HDAC9. HDAC9a is 132 amino acids shorter than HDAC9, but both isoforms contain the HDAC catalytic domain, remain capable of deacetylase activity and repress myoctye enhancer-binding factor 2-mediated transcription.