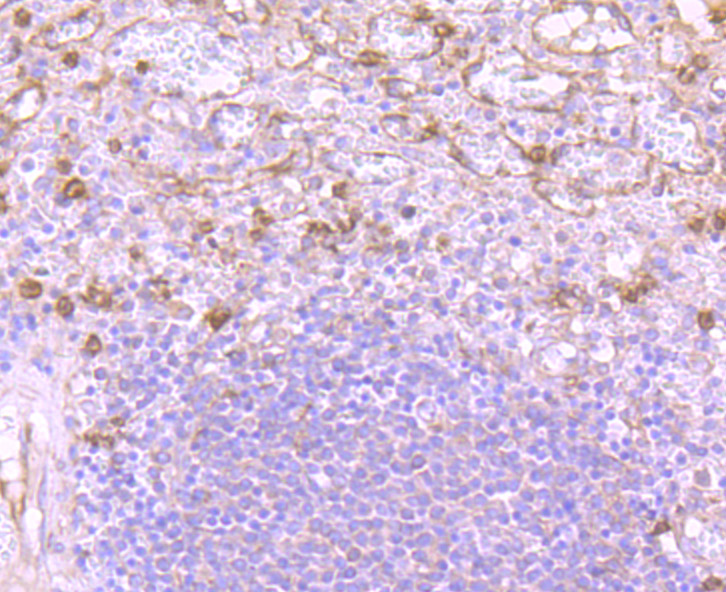
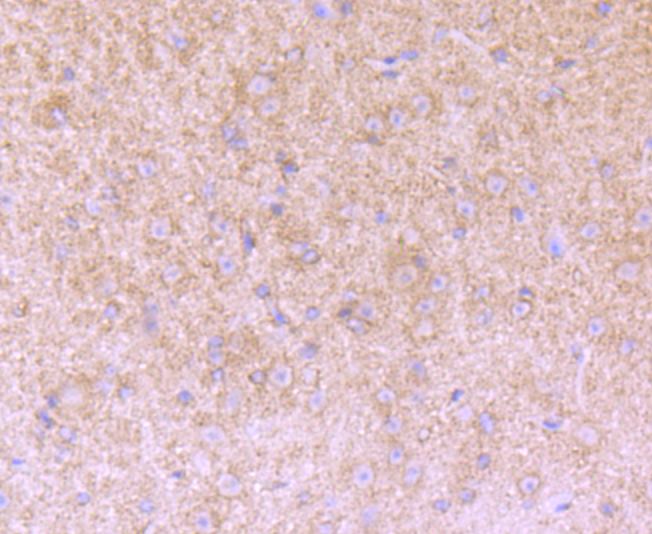
Versican (chondroitin sulfate proteoglycan 2) is a large extracellular matrix proteoglycan involved in cell growth and differentiation. Important as a structural molecule, versican creates loose and hydrated matrices during key events in development and disease. The protein contains hyaluronic acid and glycosminoglycan-binding domains, epidermal growth factor-like repeats, a lectin-like sequence and a complement regulatory protein-like domain. Splice variants differ greatly in length and degree of modification by glycosaminoglycan chains. Accumulation around smooth muscle cells in lesions of atherosclerosis, suggests a role for versican in atherogenesis. Versican, differentially expressed in human melanoma, plays a role in tumor development and may be a reliable marker for clinical diagnosis. The organization of HA- and versican-rich pericellular matrices may faciliatate migration and mitosis by diminishing cell surface adhesivity and affecting cell shape through steric exclusion and the viscous properties of HA proteoglycan gels. The human verisican gene maps to chromosome 5q14.3.