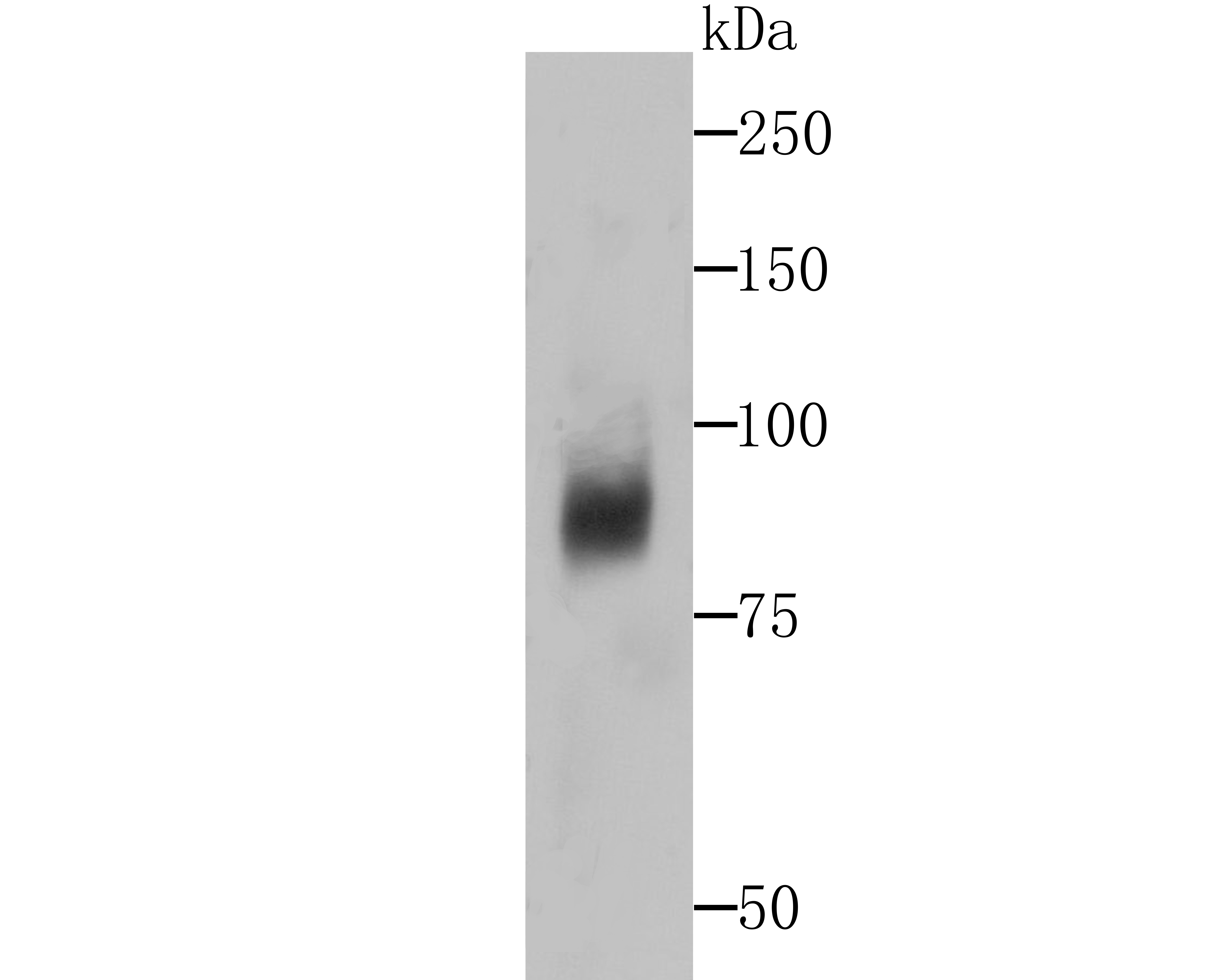
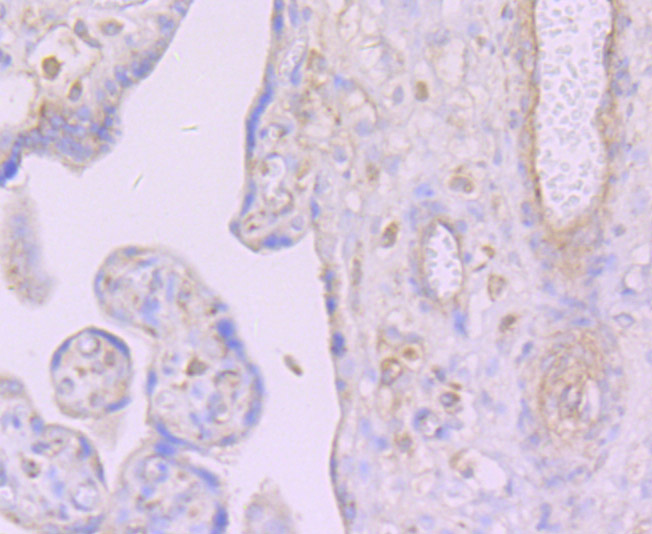
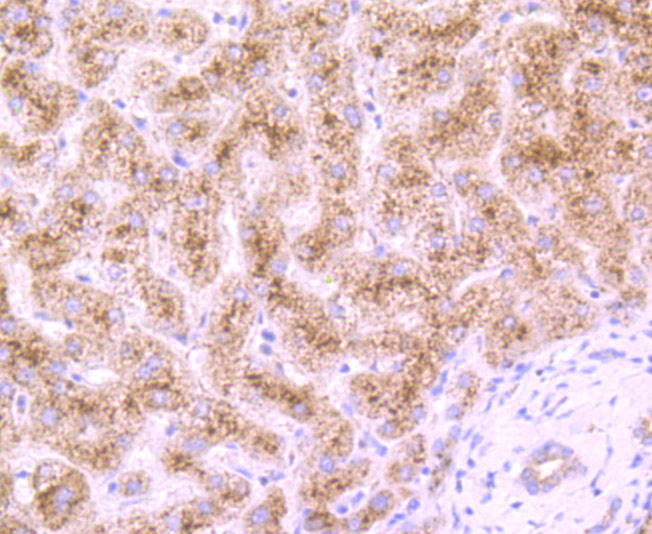
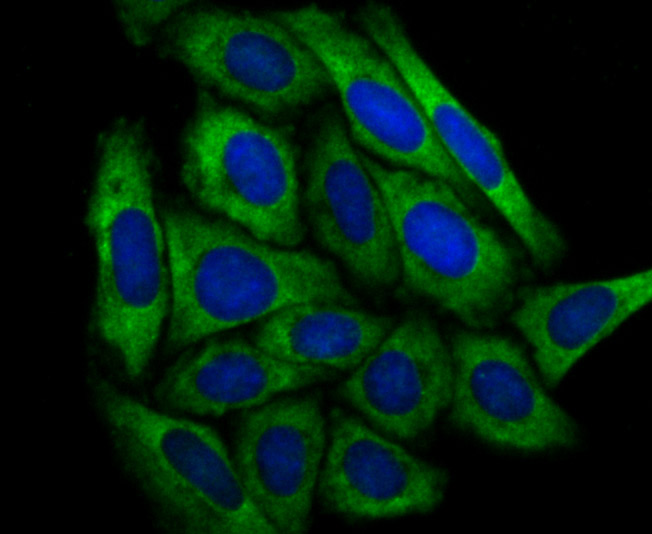
Cleavage of the 5'-cap structure is involved in the major 5'-to-3' and nonsense-mediated mRNA decay pathways. The protein complex consisting of Dcp1 and Dcp2 has been identified as the species responsible for the decapping reaction in Saccharomyces cerevisiae. In nonsense-mediated decay, the human decapping complex, made up of S. cerevisiae homologs Dcp1a and hDcp2, may be recruited to mRNAs containing premature termination codons by nonsense-mediated decay factor (Upf) proteins. hDcp2 specifically hydrolyzes methylated capped RNA to release m(7)GDP, thereby aiding in mRNA degradation. Both Dcp1a and hDcp2 colocalize in the cytoplasm. In addition, Dcp1a interacts with Smad4 forming a complex with TGFβ and BMP-4. Dcp1a and Smad4 interact directly through a EVH1/WH1 domain on Dcp1a and a proline-rich activation domain on Smad4. Smad4 is essential to nuclear translocation of Dcp1a as deletion of the Smad4-interacting domain (located in the N-terminal 100 amino acids) of Dcp1a eliminates TGFβ-induced nuclear translocation of Dcp1a.