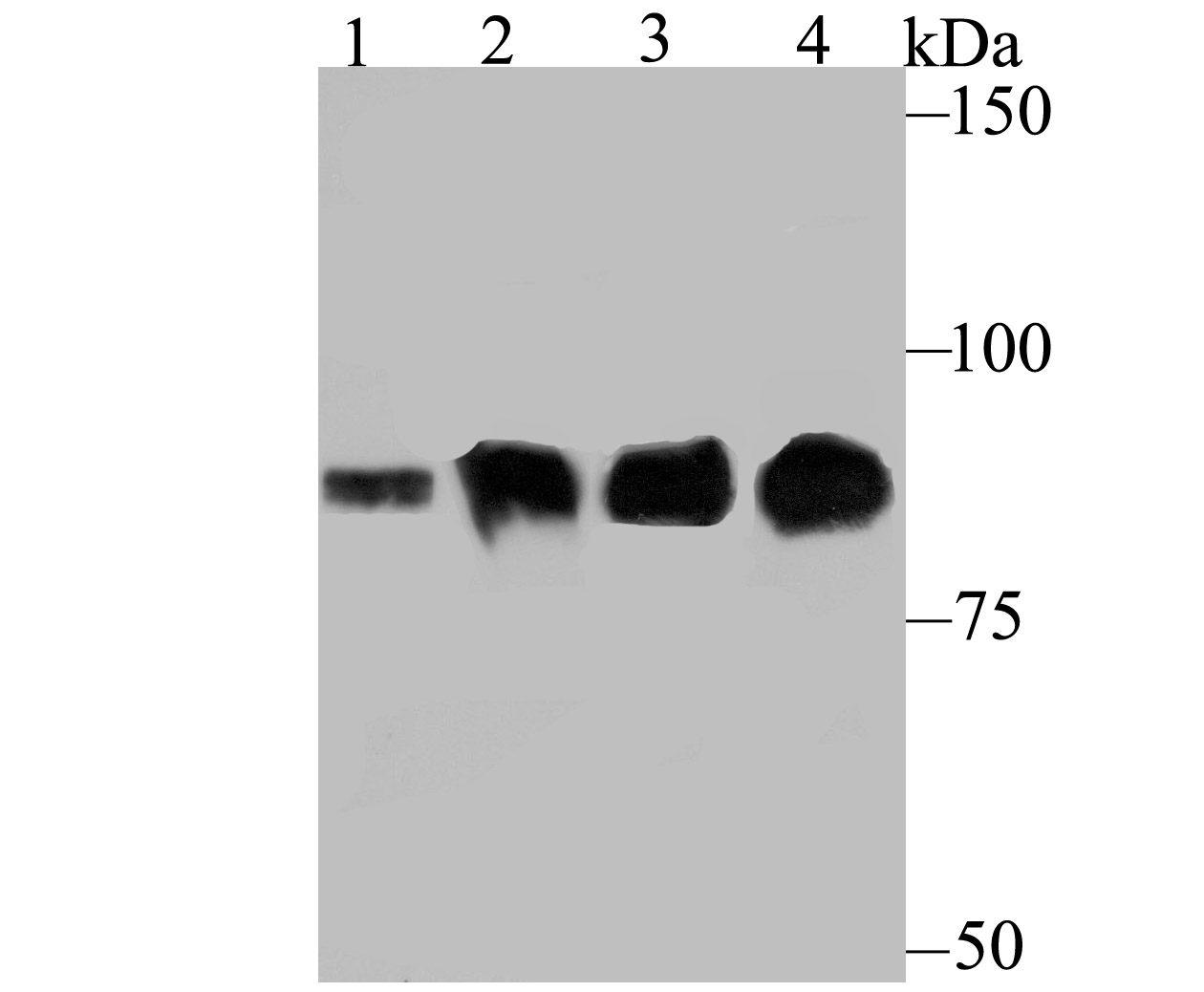
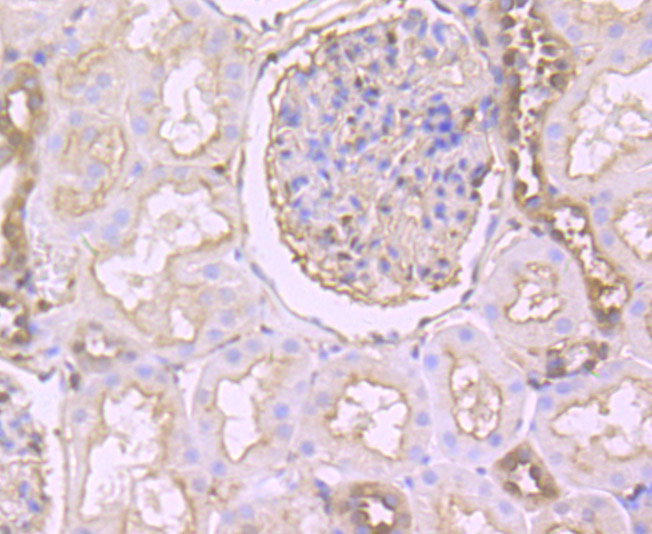
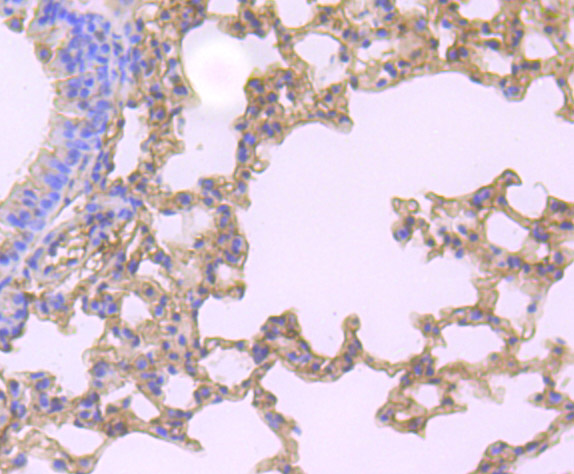
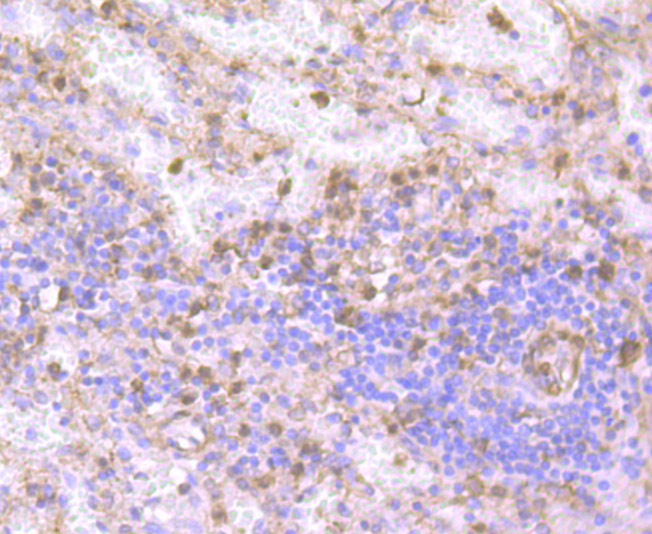
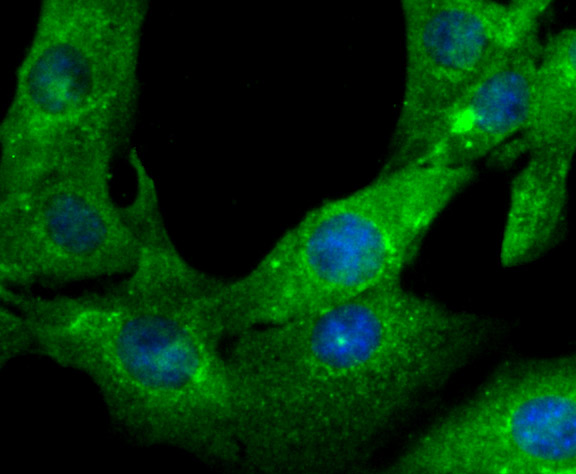
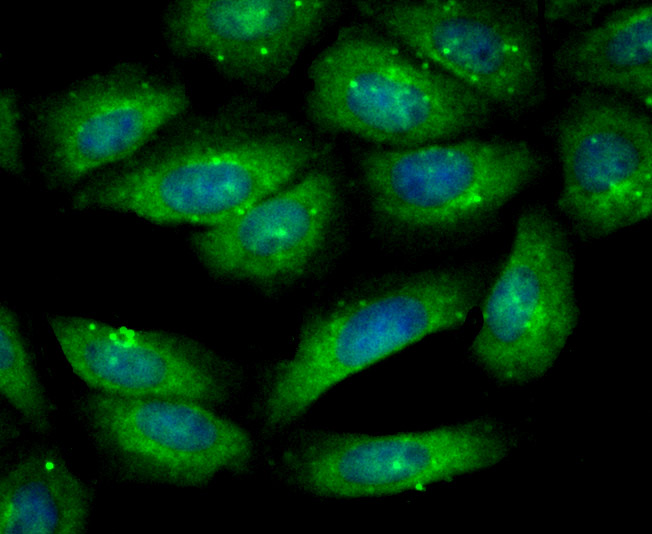
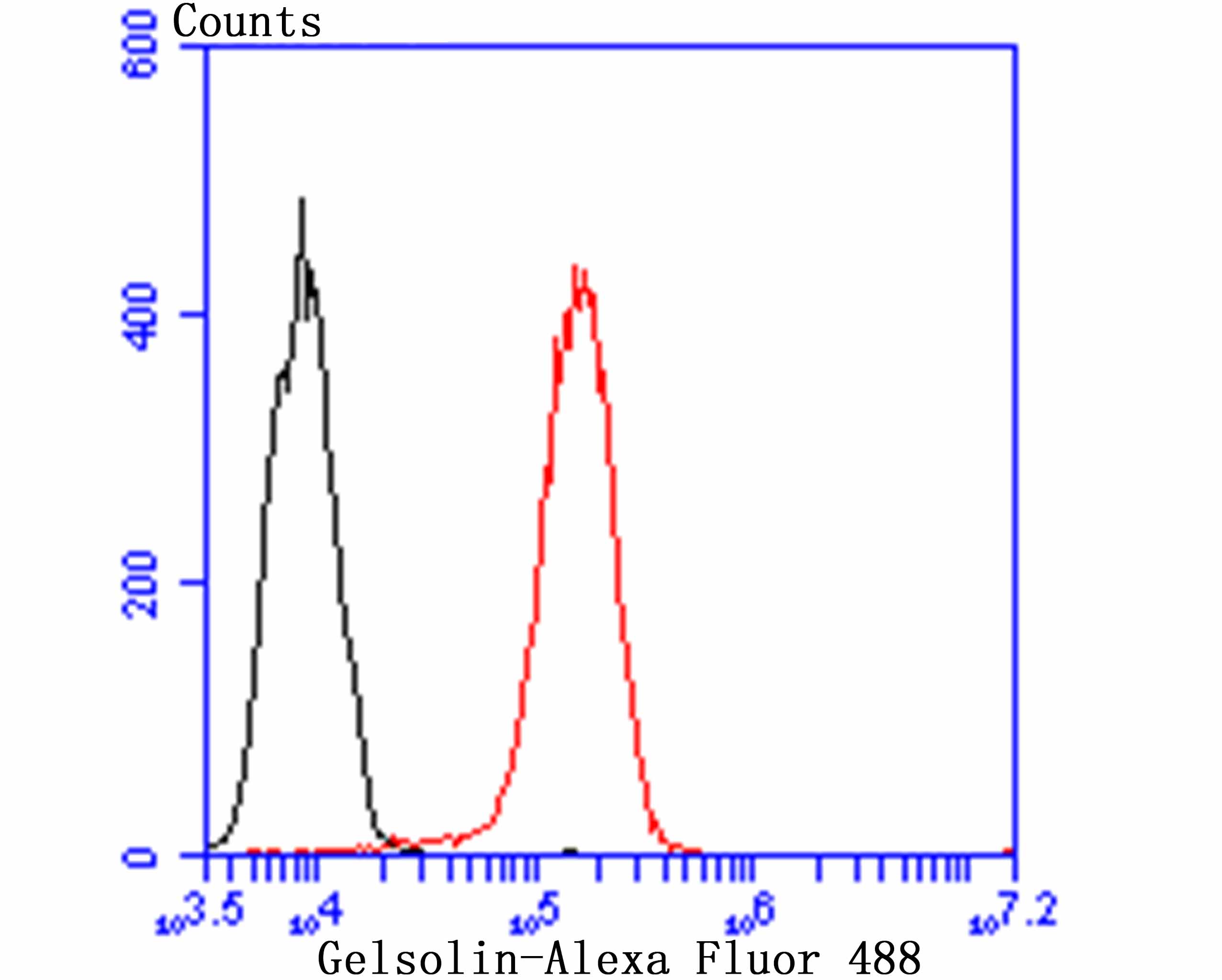
Gelsolin (also known as brevin, Actin-depolymerizing factor or ADF), a protein of leukocytes, platelets and other cells, severs Actin filaments in the presence of submicromolar calcium, thereby isolating cytoplasmic Actin gels. A calcium-independent mechanism reverses the process. A Gelsolin variant with 23 more amino-terminal amino acids is a plasma component probably involved in the clearance of Actin, the most abundant human protein, from the circulation. It has been suggested that a single gene encodes both cell and plasma gelsolins. Gelsolin may be unique in that it is made for both secretion and intracytoplasmic location. Amino acid homology was identified between Gelsolin and the amyloid of the Finnish variety of amyloidosis. The amyloid in this disorder is antigenically and structurally related to Gelsolin. Gelsolin is the principal intracellular and extracellular Actin-severing protein. Gelsolin and Gc protein together constitute the extracellular Actin-scavenger system which prevents the toxic effects of Actin release into the extracellular space under circumstances of cell necrosis.