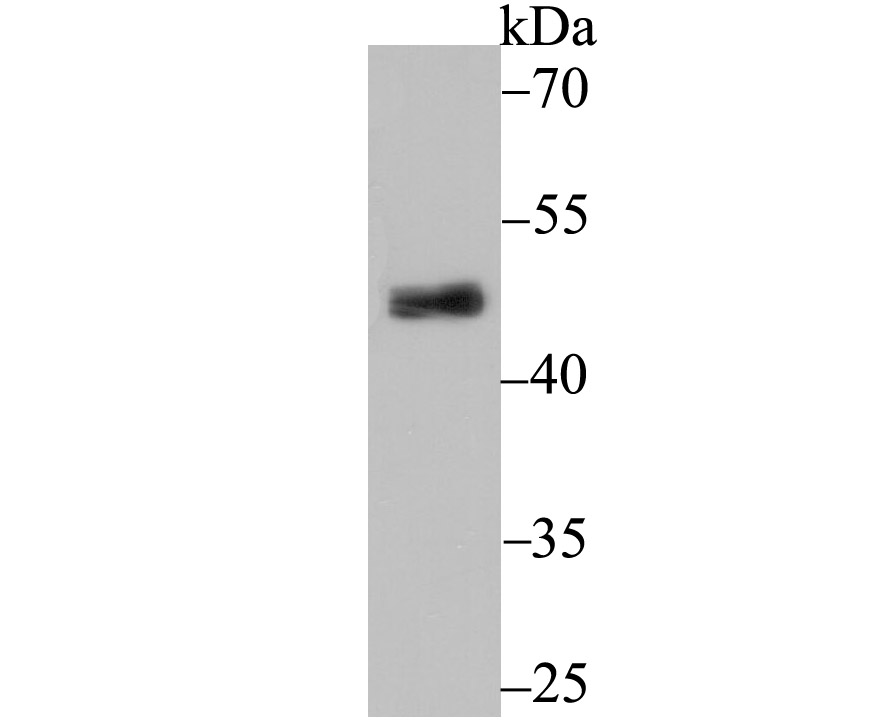
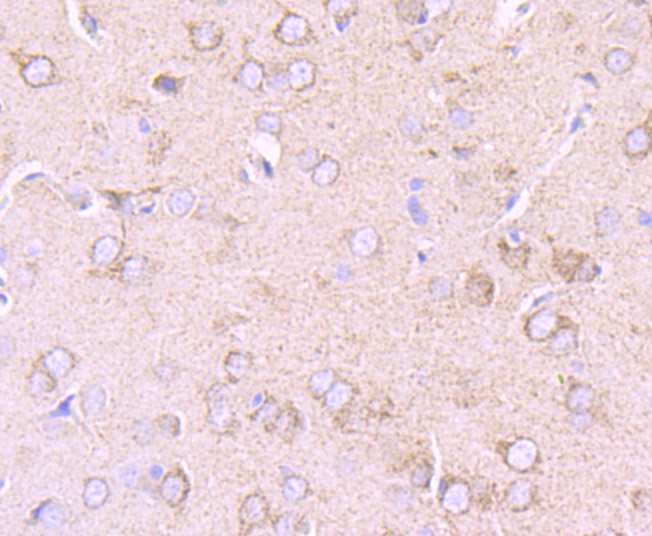
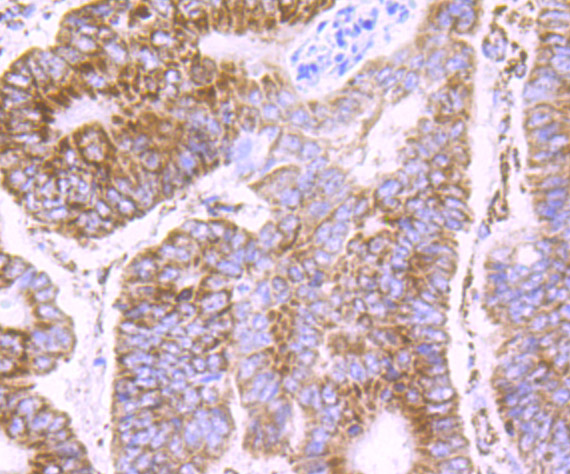
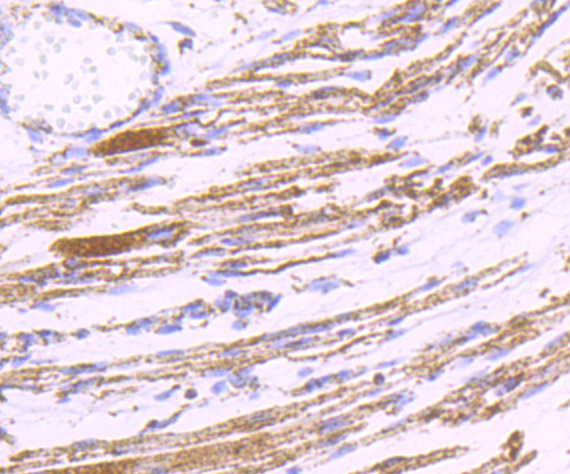
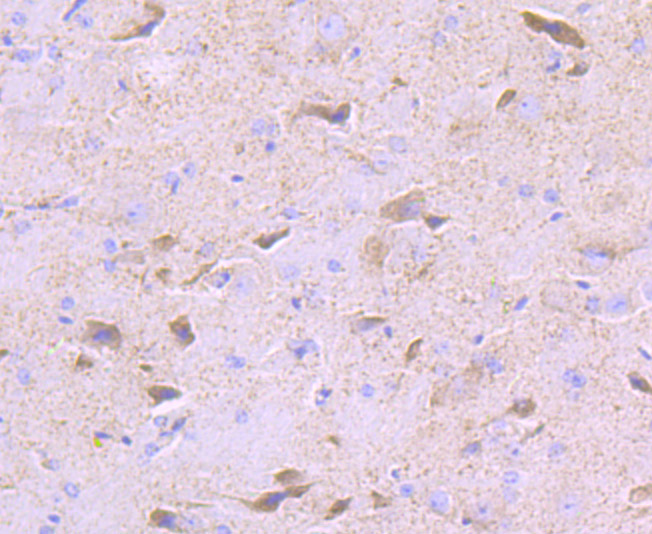
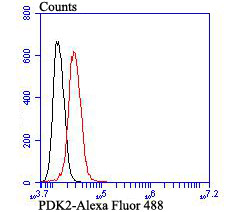
Pyruvate dehydrogenase kinase family members (PDK1, 2, 3, 4) are serine kinases that catalyze the phosphorylation of the E1α subunit of the pyruvate dehydrogenase complex (PDC). PDC activity is controlled through phosphorylation and dephosphorylation of the E1α subunit, which leads to inactivation and reactivation, respectively. The core of PDC is composed of sixty dihydrolypoyl acetyltransferase (E2) subunits that bind directly to PDK2 and enhance PDK2 kinase activity. Upregulation of PDK isoenzymes occurs during starvation conditions, rerouting acetyl-CoA generation by facilitating fatty acid oxidation. PDKs contain five conserved regions and are mechanistically similar to bacterial His-kinases, in that both require Histidine residues for activity. In mammals, transcripts for PDK2 are ubiquitously expressed with high levels in heart and skeletal muscle and decreased levels in spleen and lung.