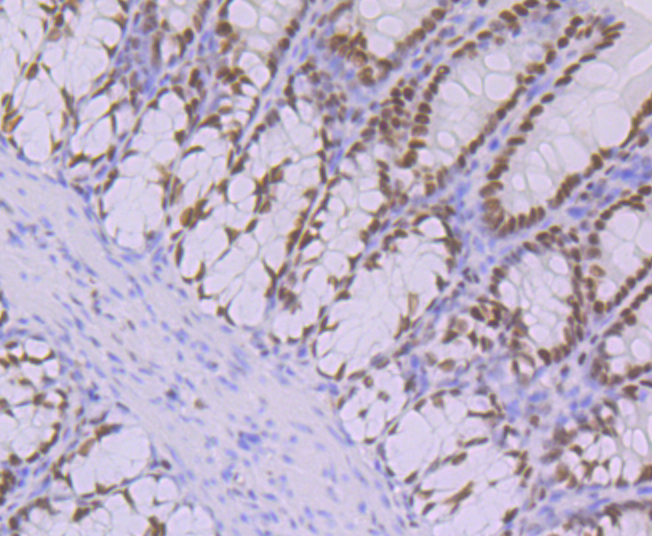
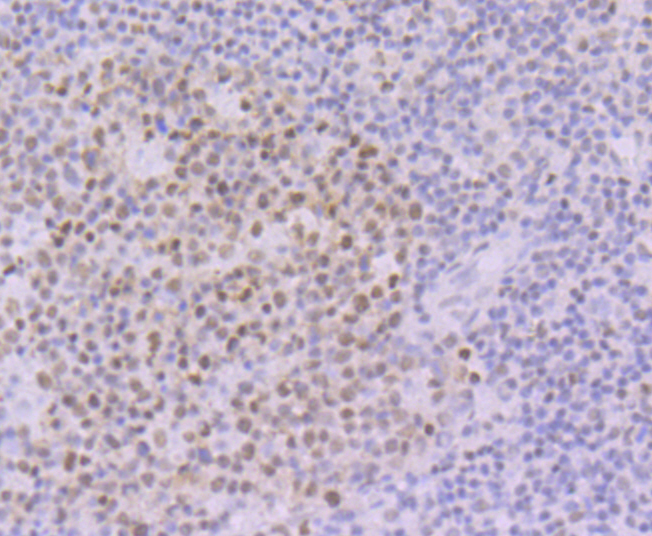
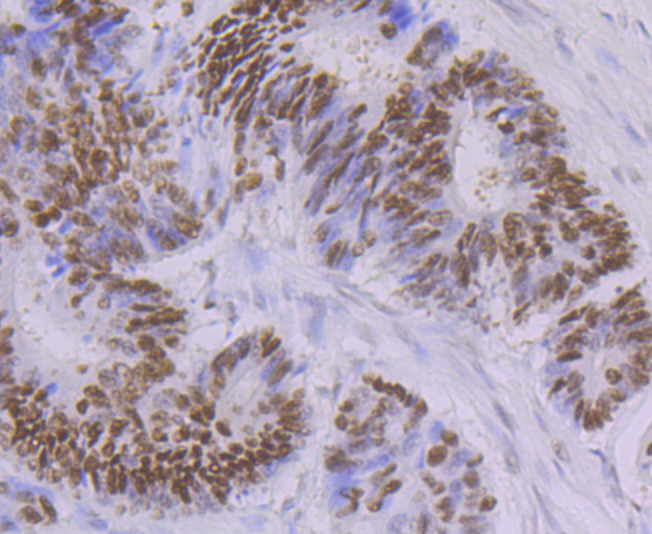
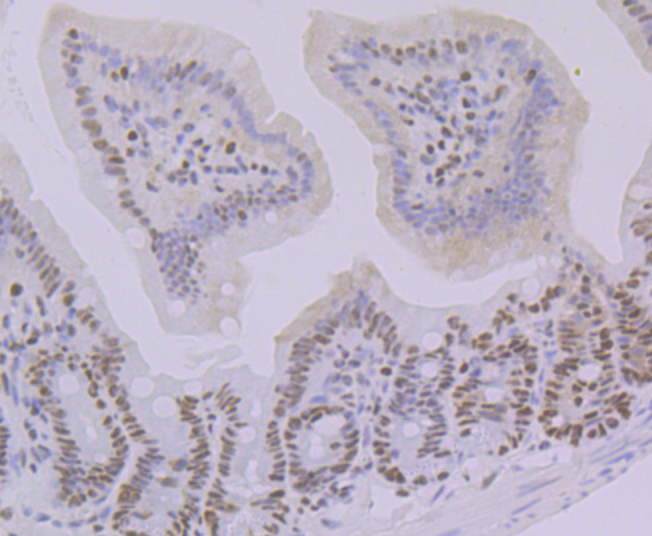
POU domain proteins contain a bipartite DNA binding domain divided by a flexible linker that enables them to adopt various monomer configurations on DNA. The versatility of POU protein operation is additionally conferred at the dimerization level. The POU dimer from the Oct-1 gene formed on the palindromic Oct factor-recognition element (PORE), which is comprised of an inverted pair of homeodomain-binding sites separated by exactly 5 bp (ATTTGAAATGCAAAT), could recruit the transcriptional co-activator OBF1. Studies of tissue-specific expression of immunoglobulin promoters demonstrate the importance of an octamer, ATTTGCAT, and the proteins that bind to it. This is a regulatory element important for tissue- and cell-specific transcription, as well as for transcription of a number of housekeeping genes. The Oct-1 gene encodes one protein, NF-A1, which is found in nuclear extracts from all cell types and thus is not specific to lymphoid cells as is the protein NF-A2, which is encoded by the Oct-2 gene.