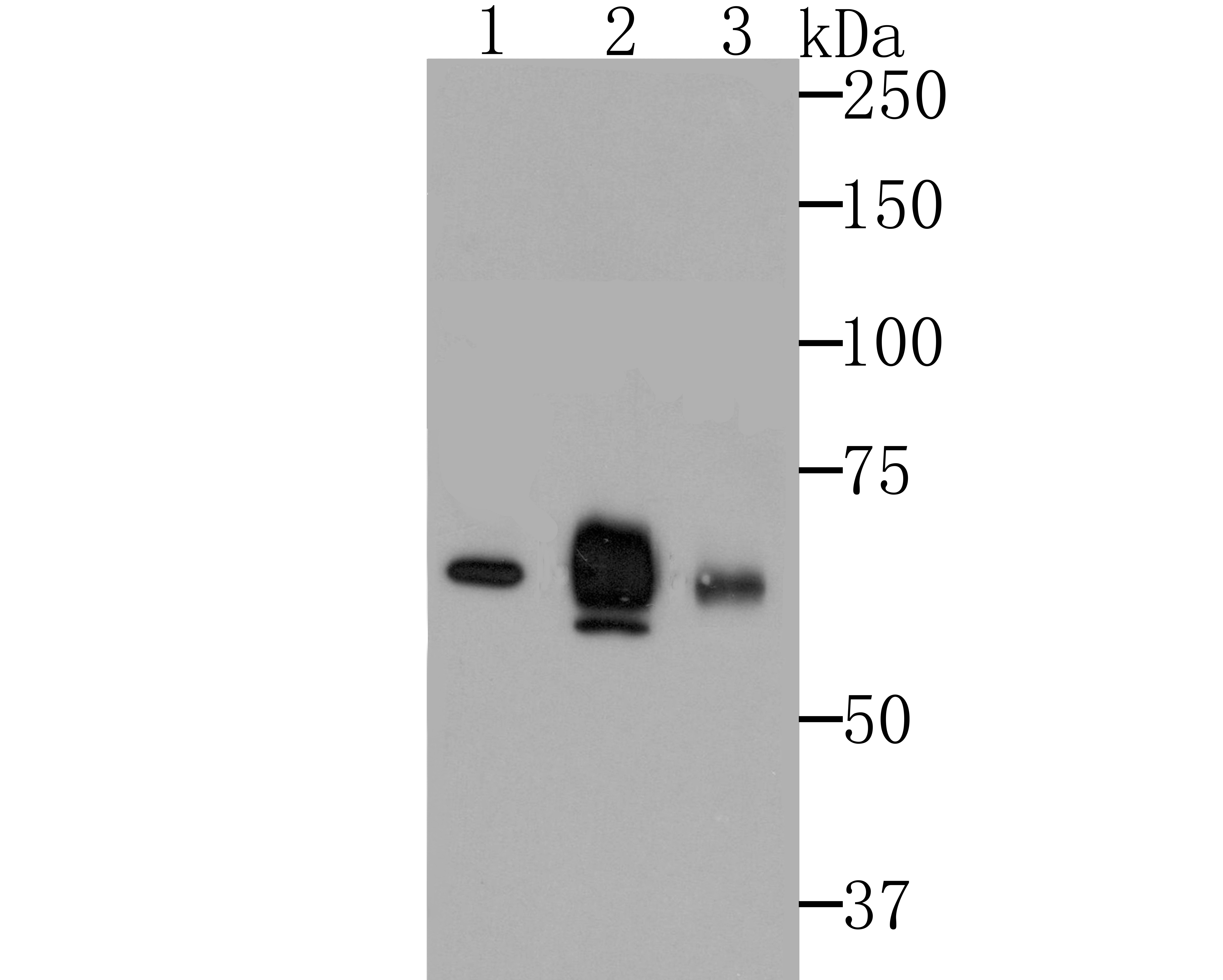
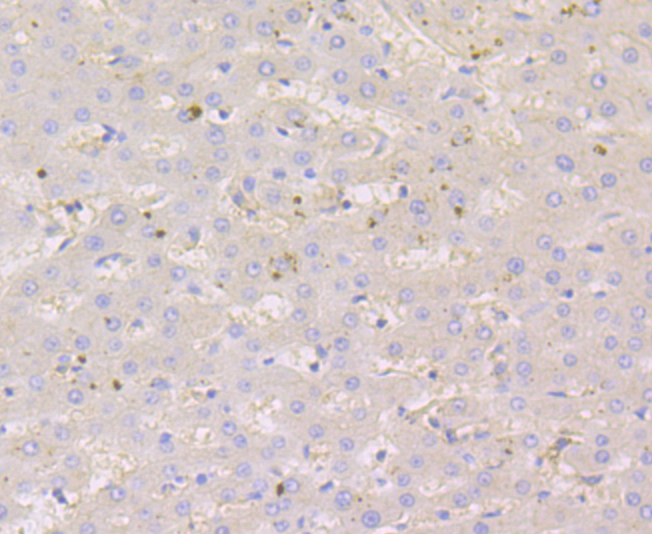
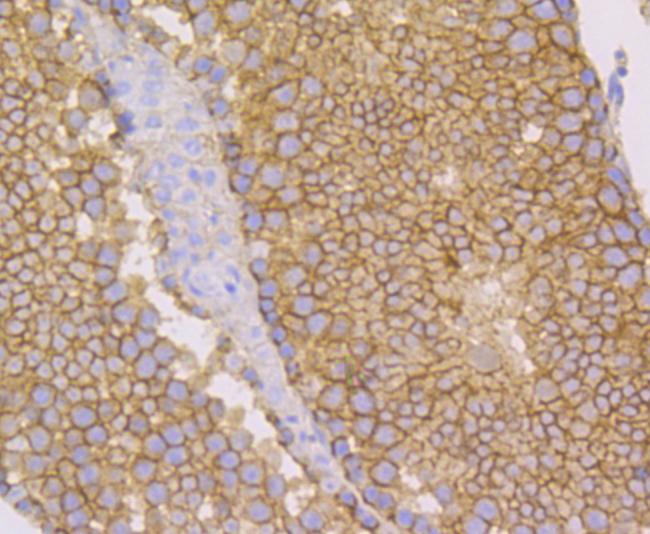
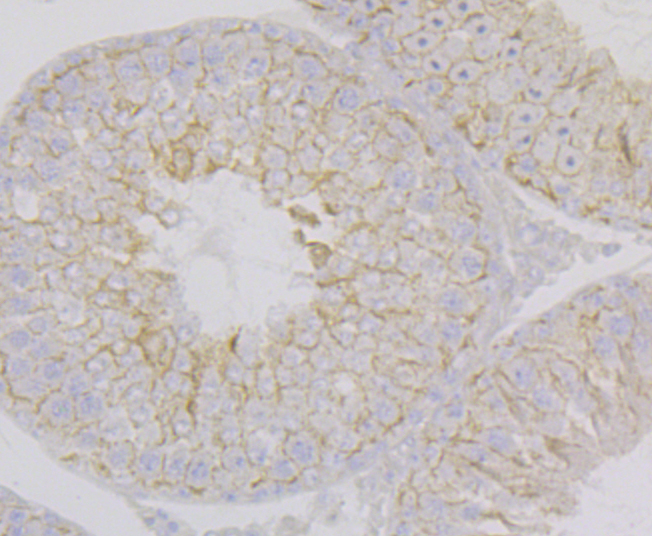
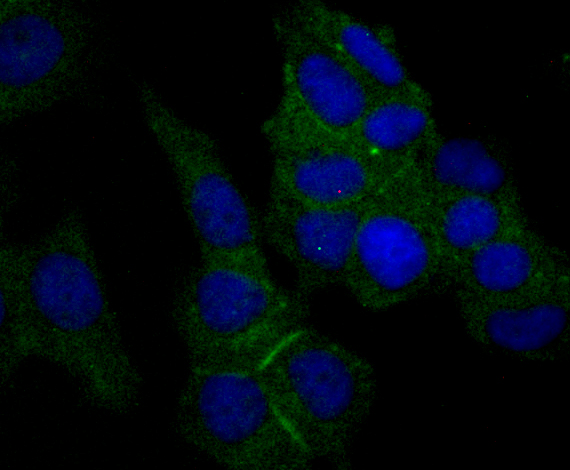
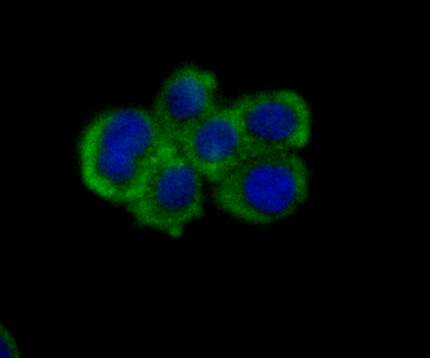
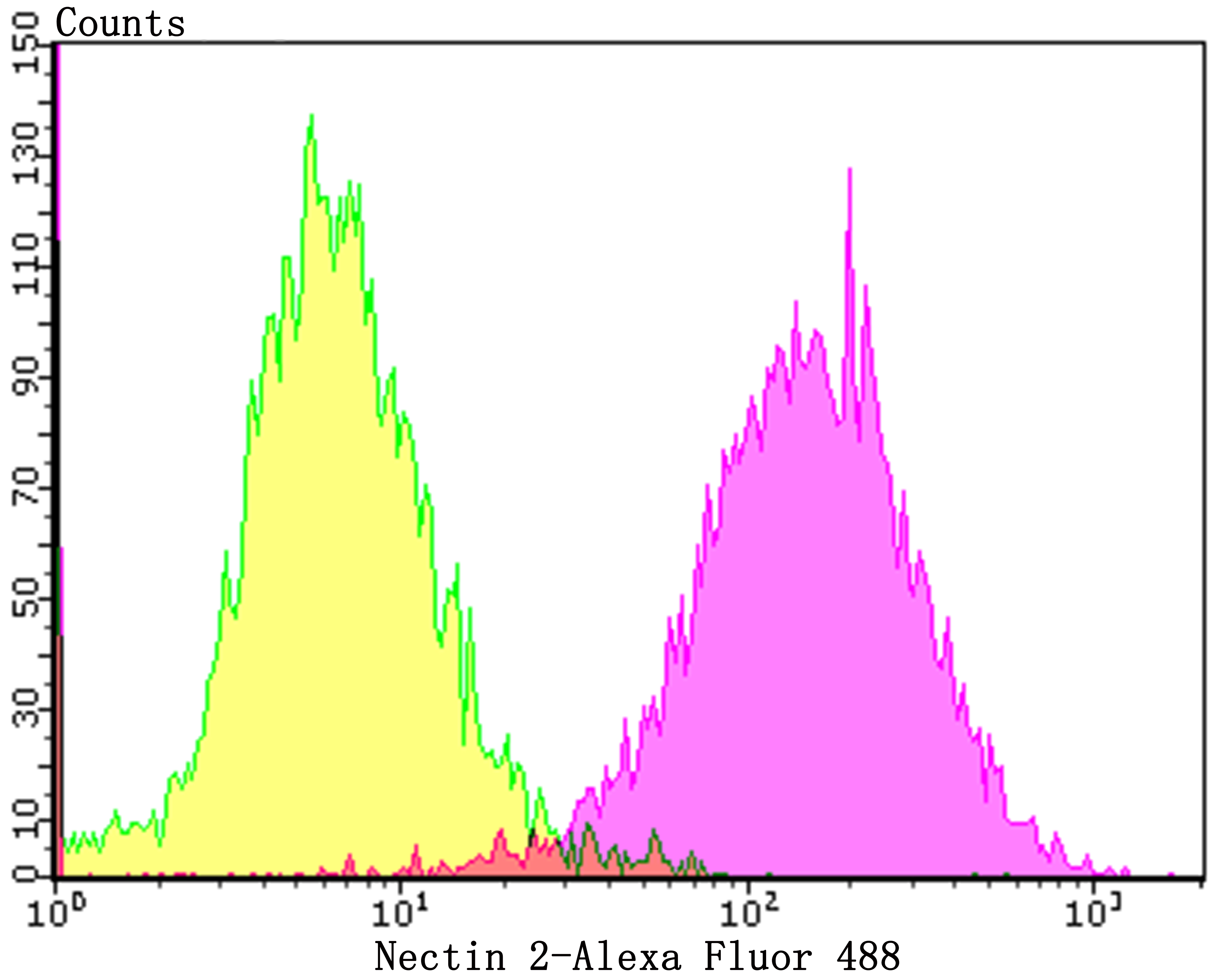
Nectin is a Ca2+-independent homophilic cell adhesion molecule that belongs to the immunoglobulin superfamily. Human nectin is identical to the poliovirus receptor-related protein (PRR) and has been identified as the α-herpesvirus entry mediator. Nectin constitutes a family consisting of at least Nectin 1, 2 and 3; each member has two or three splicing variants. Nectin 2, also designated PRR2/HveB, is ubquitously expressed, with the highest levels of expression in some human neuronal cell lines, fibroblastic cells, keratinocytes and primary activated T lymphocytes. Nectin 2 has two splicing variants, Nectin 2α (short form) and 2δ (long form). Both Nectin 2α and 2δ have a C-terminal conserved motif (E/A-X-Y-V). This motif interacts with the PDZ domain of the F-Actin-binding protein afadin, through which it is linked to the Actin cytoskeleton. The extracellular regions of the splicing variants are identical, but their transmembrane regions and cytoplasmic regions are unique. Nectin 2 mediates the entry of three mutant herpes simplex virus type 1 (HSV-1) strains that do not use HveA as co-receptor, but not wildtype HSV-1 strains. Nectin 2 also mediates the entry of HSV-2 and pseudorabies virus, but not bovine herpes virus type 1. Nectin 2δ is tyrosine phosphorylated in response to cell-cell adhesion.