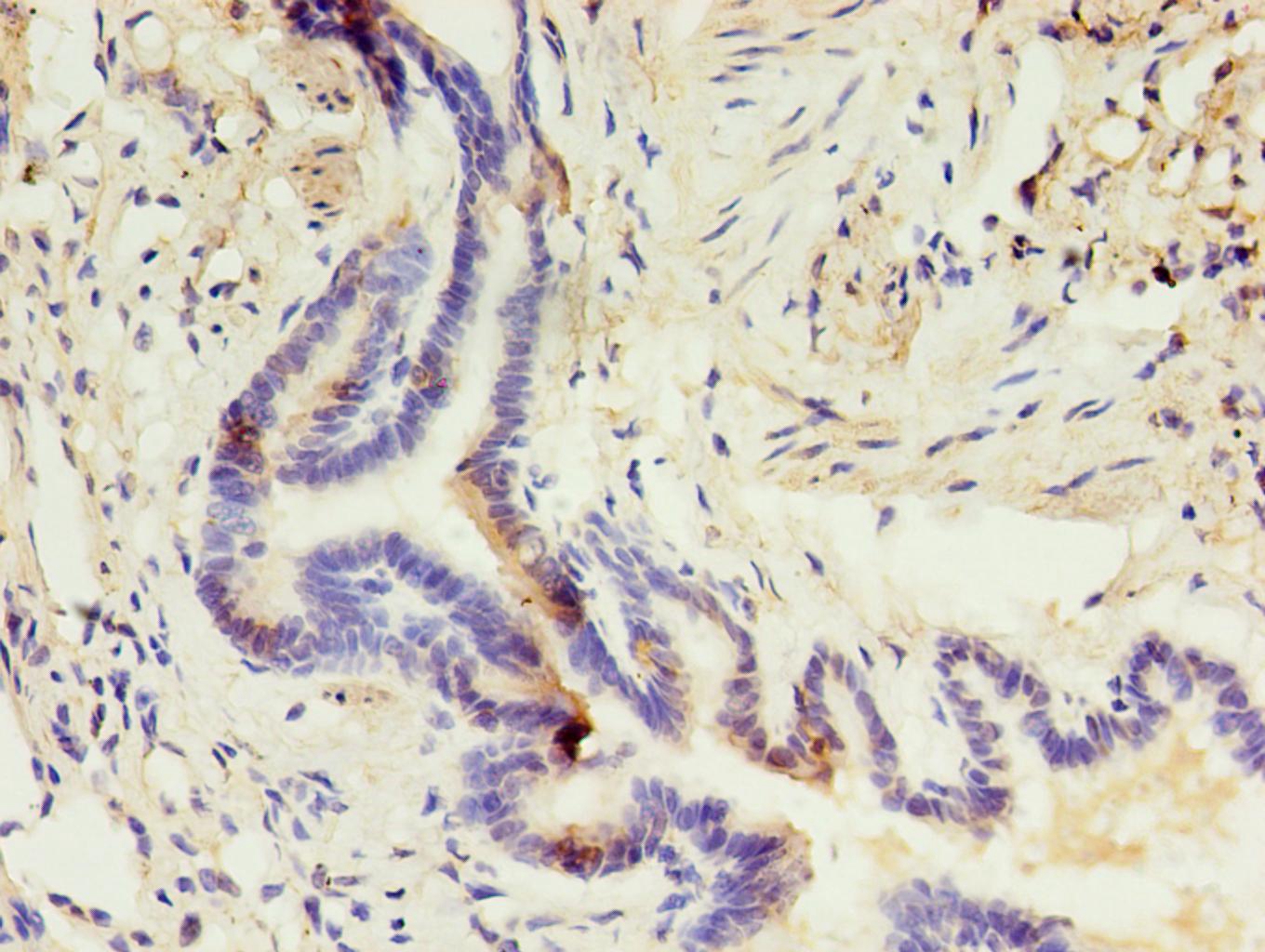
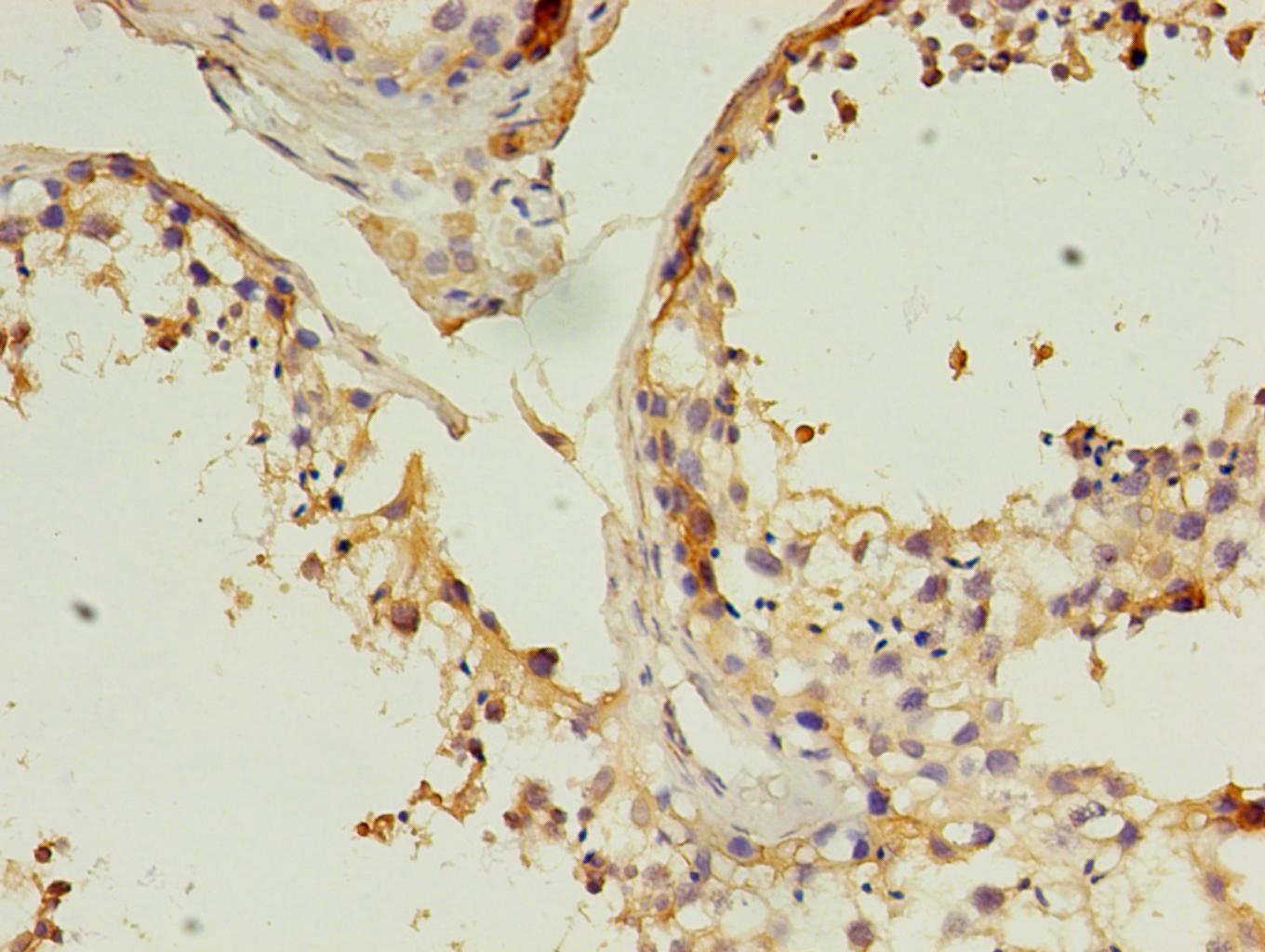
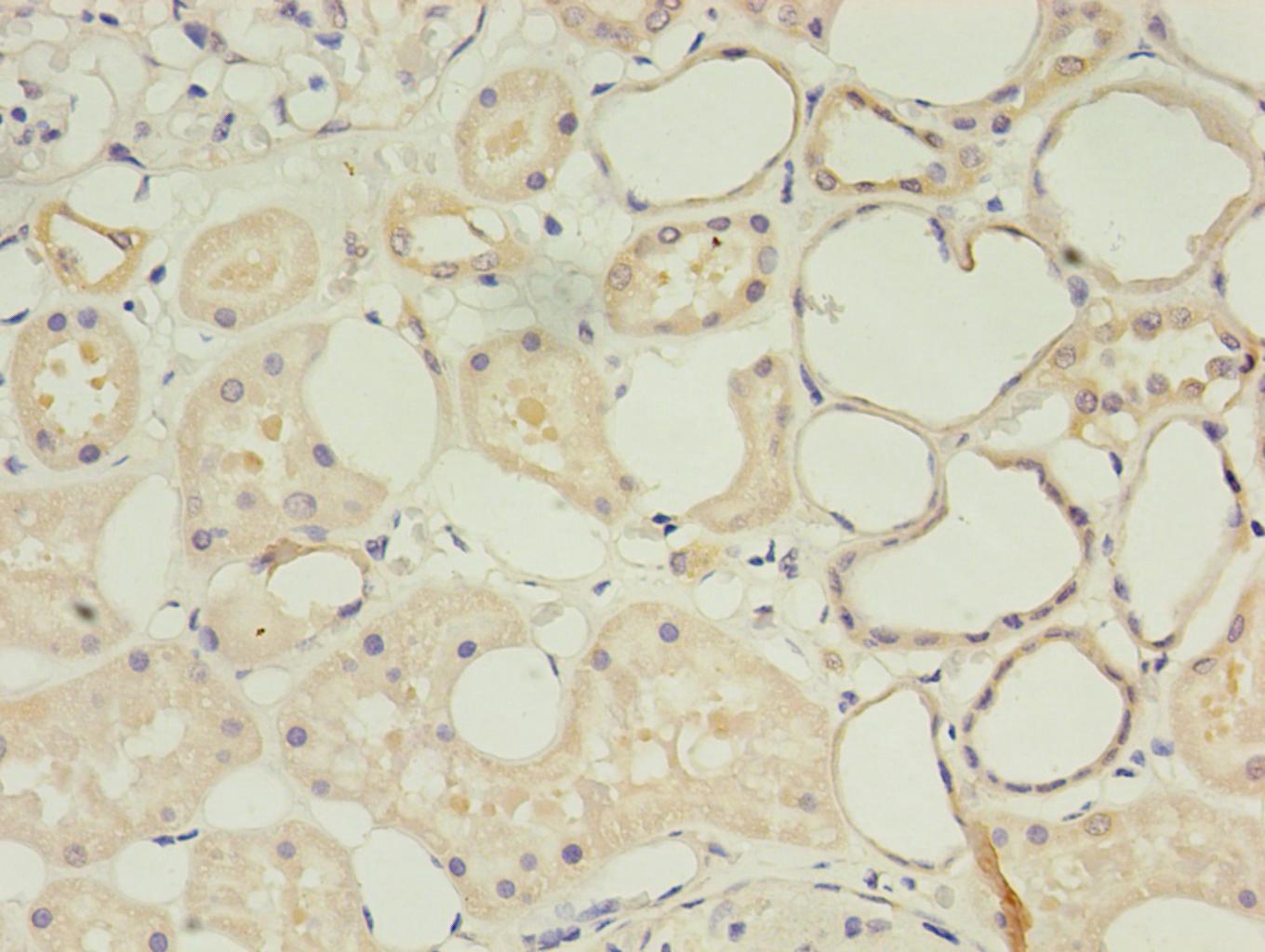
As a component of the innate immune system, the cell surface glycoprotein CD14 is a myelomonocytic differentiation antigen preferentially expressed on monocytes, macrophages, and activated granulocytes. CD14 exists as two forms, either anchored into the membrane by a GPI-anchor tail (mCD14) or present as a soluble form (sCD14) in normal serum and body fluids. CD14 was first described as a pattern recognition receptor for lipopolysaccharide (LPS) and a variety of ligands derived from different microbial sources, along with the co-receptors Toll-like receptor TLR 4 and MD-2. The binding of CD14 and LPS depends on the presence and catalytic activity of lipopolysaccharide-binding protein (LBP). Besides its endotoxin signaling function, CD14 has been proposed to be involved in various biological processes, including transportation of other lipids, cell-cell interaction during different immune responses, as well as recognition of apoptotic cells. Multiple transcript variants resulting from alternative splicing encode the same isoform of CD14.