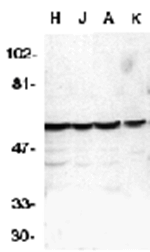
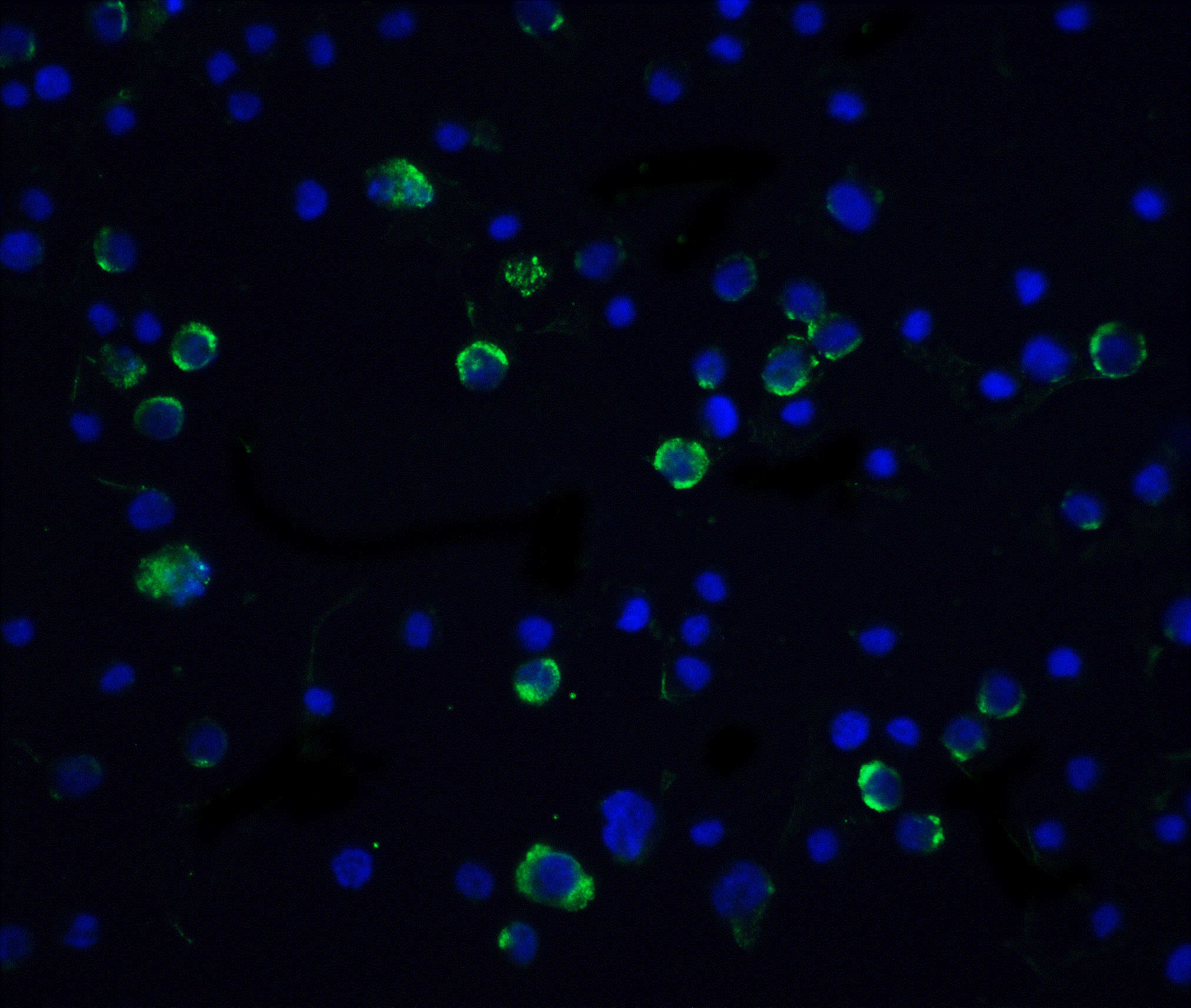
Apoptosis is related to many diseases and induced by a family of cell death receptors and their ligands. Cell death signals are transduced by death domain (DD)- containing adapter molecules and members of the ICE/CED-3 protease family. A novel ICE/CED-3 protease was identified recently, designated FLICE2 and Mch4 and renamed as caspase-10. Caspase-10 has two death effector domains (DEDs) that bind to the DED in the adapter molecule FADD and recruits both TNFR1 and CD95 to form complexes with these receptors. Caspase-10 is therefore involved in the CD95 and TNFR1 induced apoptosis. Caspase-10 cleaves and activates caspase-3, -4, -6, -7, -8 and -9, which causes the proteolytic cleavage of many key proteins such as PARP. Cleavage of PARP occurs in many different systems during apoptosis and is the hallmark of programmed cell death. Caspase-10 is expressed in many tissues and cell lines.