INTENDED USE: One Step Test for Novel Coronavirus (2019-nCoV) IgM/IgG Antibody (Colloidal Gold) is intended for the qualitative detection of 2019-Novel
Coronavirus IgM and IgG antibody in serum, plasma, fingertip blood or whole blood samples of pneumonitis patients or suspected cases.
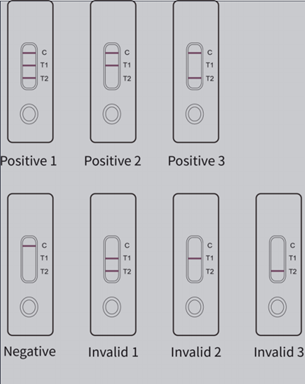
PRINCIPLE: The test uses mixed recombinant 2019-nCoV nucleocapsid protein (N protein) and spike protein (S protein) both conjugated with colloidal gold and anti-human IgM and IgG antibody coated on different test lines respectively. After the samples has been applied to the test strip, the gold-labelled recombinant 2019-nCoV N protein and S protein will bind with 2019-nCoV IgM or IgG antibody in sample and form marked antigen-antibody complexes. These complexes move to the test card detection zone by capillary action. Then marked antigen-antibody complexes will be captured on different test lines by anti-human IgM and IgG antibody resulting in purplish red streaks on the test lines. The color intensity of each test line increases in proportion to the amount of 2019-nCoV IgM and IgG antibody in sample.
SUMMARY: that has not been previously identified in humans. The disease is primarily spread between people via respiratory droplets from infected individuals when they cough or sneeze. Time from exposure to onset of symptoms is generally between 2 and 14 days. The disease may initially present with few or no symptoms, or may
develop into fever, coughing, shortness of breath, pain in the muscles and tiredness. Further development may include pneumonia and acute respiratory distress syndrome.
Detection of 2019-nCoV IgM and IgG antibodies in human blood can be used as an auxiliary means for early screening of COVID-19. 2019-nCoV IgM antibody could be detected in patient blood in 3-5 days after onset and IgG could be detected in 7 days after onset. However, the trend of IgM and IgG changes in different cases is not exactly the same. As it is a novel disease diagnosis and treatment of which are being explored, please refer to the latest guidelines for diagnosis and treatment of COVID-19.
CONTENTS: 1. A kit contains:
Package specifications: 100 tests/box, 50 tests/box, 25 tests/box, 5 tests/box,3 tests/box, 1 test/box.
1) Getein Novel Coronavirus (2019-nCoV) IgM/IgG Antibody test card in a sealed pouch with desiccant
2) Disposable pipet
3) Sample diluent
4) User manual: 1 piece/box
Note: Do not mix or interchange different batches of kits.
2. A test card consists of:
A plastic shell and a reagent strip composed of a sample pad, a colloidal gold pad (coated with recombinant 2019-nCoV N protein and S protein),
nitrocellulose membrane with two test lines (these two lines are coated with anti-human IgM and IgG antibody respectively), the control line (coated with anti recombinant protein tag protein), absorbent paper and liner.
3. Sample diluent composition:Phosphate buffered saline, proteins, detergent, preservative, stabilizer.
PRECAUTIONS: 1. Do not reuse the pipet to avoid cross contamination.2. Do not open pouches until ready to perform the test to protect the test cards from getting damp from being exposed in air for too long.3. The test cards can be stored in room temperature with sealed pouches.And the test cards stored in low temperature should reach room temperature before testing.
4. Sodium azide is used as preservative which may react with copper drainage pipe or lead drainage pipe and cause explosive substance.Please dispose with the preservative properly according to relevant local regulations.
5. There should be appropriate bio-safety assurance procedure for infectious sources or potential infectious sources. Some relevant precautions are
showed below:
(1) Wear disposable gloves to deal with samples, or sterilize reagents.
(2) Sterilize spilled samples or reagents with sanitizer.
(3) Sterilize and dispose all of samples, reagents and potential
contaminant with relevant local regulations.
SPECIMEN COLLECTION AND PREPARATION:
1. Sample should be human serum, plasma, fingertip blood and whole blood, other body fluid and samples may cause incorrect or inaccurate results.
2. Venous blood should be collected under sterile condition at any time of a day.
3. Suggest using serum or plasma for better results.
4. Heparin, sodium citrate and EDTA can be used as anticoagulant for
plasma, fingertip blood and whole blood sample.
5. Serum, plasma, fingertip blood or whole blood sample should be tested within 4 hours after blood collection in room temperature. If testing will be
delayed, serum and plasma may be stored up to 5 days at 2-8 ℃ or stored for 6 months at -20 ℃ before testing (fingertip blood and whole blood sample may be stored up to 3 days at 2-8 ℃). Do not heat the samples and discard hemolyzed samples.
6. Bring all samples to room temperature (15-30 °C) before use. Refrigerated or frozen sample should reach room temperature and be homogeneous before testing. Avoid multiple freeze-thaw cycles.
7. SAMPLE VOLUME: 10 μl of serum and plasma sample, 20 μl of fingertip
blood and whole blood sample.
TEST PROCEDURE:
Read the manual carefully before using and operate according to the manual to avoid incorrect results.
1. Collect specimens according to user manual.
2. Test card, sample and reagent should reach to room temperature (15-30 ℃) before test.
3. Remove the test card from the sealed pouch immediately before use.Label the test card with patient or control identification.
4. Put the test card on a clean table, horizontally placed.
5. Using sample transfer pipette, deliver sample (10 μl of serum and plasma sample, 20 μl of fingertip blood and whole blood sample) into the sample
port on the test card. Then add three drops of sample diluent immediately.
6. Read the results visually in 10-20 min.