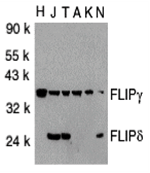
Apoptosis is related to many diseases and induced by a family of cell death receptors and their ligands. Cell death signals are transduced by death domain (DD)-containing adapter molecules and members of the ICE/CED-3 protease family. Caspases-8 (FLICE) and -10 (FLICE2) are two pivotal members in the ICE/CED-3 protease family. FLICE-inhibitory proteins were identified in virus and human and designated v-FLIPs and c-FLIPs, respectively. The human FLIPs were also cloned by several labs independently and termed Casper , I-FLICE, FLAME-1, CASH, CLARP and usurpin. FLIP contains two death effector domains (DEDs) and a caspase-like domain. FLIP interacts with adapter protein FADD and caspase-8 and 10, and potently inhibits apoptosis induced by all known death receptors CD95, DR3, TRAIL-R and TNFR1. Four splice variants of c-FLIPs have been identified and termed FLIPalpha, beta, gamma, and delta, respectively.