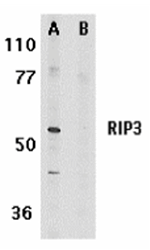
Certain serine/threonine protein kinases, such as ASK1, RIP, DAP, and ZIP kinases, are mediators of apoptosis. Receptor interacting proteins including RIP and RIP2/RICK mediate apoptosis induced by TNFR1 and Fas, two prototype members in the death receptor family. A novel member in the RIP kinase family was recently identified and designated RIP3. RIP3 contains N-terminal kinase domain but, unlike RIP or RIP2, lacks the C-terminal death or CARD domain. RIP3 binds to RIP and TNFR1, mediates TNFR1 induced apoptosis, and attenuates RIP and TNFR1 induced NF-κB activation. Overexpression of RIP3 induces apoptosis and NF-κB activation. The messenger RNA of RIP3 is expressed in a subset of adult tissues.