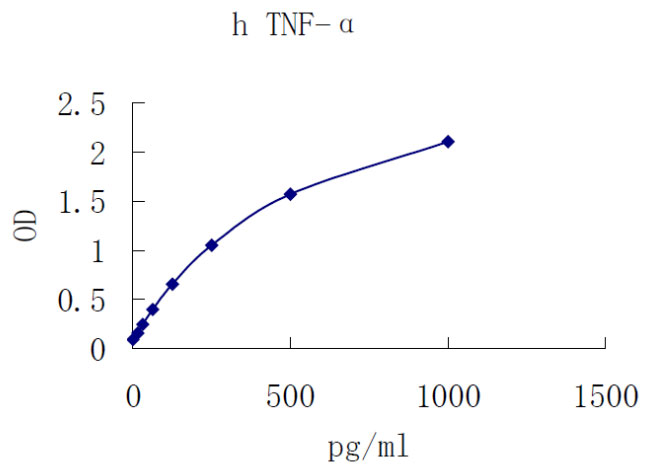
The prototype ligand of the TNF superfamily, TNF-α/TNFSF1A, is a pleiotropic cytokine that plays a central role in inflammation and apoptosis (1-4). Human cells known to express TNF-α include B cells, colonic columnar epithelial cells, NK and CD3+CD56+ hepatic natural T cells, macrophages, monocytes and monocyte-derived dendritic cells, CD4+ and CD8+ T cells, mast cells, neutrophils, keratinocytes, plasma cells, and adipocytes.
It is synthesized as a 26 kDa, type II transmembrane protein that is 233 amino acids (aa) in length (4, 5). It contains a 30 aa cytoplasmic domain, a 26 aa transmembrane segment, and a 177 aa extracellular region (6, 7). TNF-α is assembled intracellularly to form a transmembrane, non-covalently-linked homotrimeric protein. The 157 aa residue soluble form of TNF-α (sTNF-α is released from the C-terminus of the transmembrane protein through the activity of TNF-α-converting enzyme (TACE), a membrane-bound disintegrin metalloproteinase (8, 9).
TNF-α is reported to promote inflammatory cell infiltration by upregulating leukocyte adhesion molecules on endothelial cells, serve as a chemotactic agent for monocytes, and activate phagocyte killing mechanisms (10). Deficiencies in either TNF-α or its receptors can increase susceptibility to infection by intracellular pathogens (11 - 12). TNF- may also play a role in lymphoid tissue development. Knockout mice lack splenic B cell follicles and the ability to form germinal centers (13, 14). Other potential physiological roles for TNF-α and its receptors include regulating the differentiation of hematopoietic stem and progenitor cells (15 - 17).
TNF-α has been implicated in a number of pathophysiological processes. It is associated with unregulated pro-inflammatory activity and is thought to be a critical mediator of endotoxin-induced septic shock (18). Cachexia (or whole body wasting) has also been associated with long-term circulating TNF-α. Other disorders with potential TNF-α involvement include asthma), type 2 diabetes, Crohn
Kwon, B. et al. (1999) Curr. Opin. Immunol. 11:340.
Idriss, H.T. and J.H. Naismith (2000) Microsc. Res. Tech. 50:184.
Sedgwick, J.D. et al. (2000) Immunol. Today 21:110.
MacEwan, D.J. (2002) Brit. J. Pharmacol. 135:855.
Pennica, D. et al. (1984) Nature 312:724.
Wang, A.M. et al. (1985) Science 228:149.
Ishisaka, R. et al. (1999) J. Biochem. 126:413.
Kriegler, M. et al. (1988) Cell 53:45.
Moss, M.L. et al. (1997) Nature 385:733.
Wang, C.Y. et al. (1998) Science 281:1680.
Peschon, J.J. et al. (1998) J. Immunol. 160:943.
Wellmer, A. et al. (2001) Infect. Immun. 69:6881.
Trevejo, J.M. et al. (2001) Proc. Natl. Acad. Sci. USA 98:12162.
Kasahara, S. et al. (2003) J. Virol. 77:2469.
Pasparakis, M. et al. (1996) J. Exp. Med. 184:1397.
Taniguchi, T. et al. (1997) Lab. Invest. 77:647.
Jacobsen, F.W. et al. (1994) Proc. Natl. Acad. Sci. USA 91:10695.
Zhang, Y. et al. (1995) Blood 86:2930.