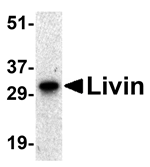
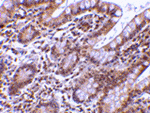
Apoptosis, or programmed cell death, is related to many diseases, such as cancer. Apoptosis is triggered by a variety of stimuli including members in the TNF family and prevented by the inhibitor of apoptosis (IAP) proteins. IAP proteins form a conserved gene family that binds to and inhibits cell death proteases. A novel member in the IAP protein family was recently identified and designated Livin and KIAP for kidney IAP. Livin/XIAP contains a single baculoviral IAP repeat (BIR) domain and a RING finger domain and has two isoforms termed Livin-alpha and Livin-beta. Transfection of Livin in cells resulted in protection from apoptosis induced by FADD, BAX, RIP, RIP3 and DR6. Livin has direct interaction with several caspases including caspase-3, -7, and -9. Livin inhibits the activation of caspase-9 induced by Apaf-1, cytochrome c, and dATP. The two isoforms of Livin appear to have different functions and tissue distributions.