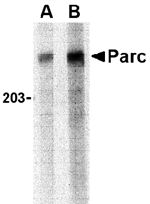
The continued localization of p53 to the nucleus is essential for its function as a tumor suppressor. PARC, a large, Parkin-like ubiquitin ligase has recently been identified as a cytoplasmic anchor protein in p53-associated protein complexes. In the absence of stress, PARC inactivation results in nuclear localization of p53 and activation of p53-dependent apoptosis, while overexpression of this protein promoted cytoplasmic sequestration of p53. Surprisingly, PARC knockout mice were viable and exhibited no obvious phenotype, suggesting that other proteins, such as the highly related cullin family of E3 ubiquitin ligases, may perform similar functions in the absence of PARC. Additionally, it has been suggested that p53 binding to PARC may serve to control PARC function.