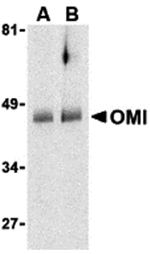
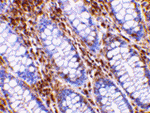
Inhibitor of apoptosis proteins (IAPs) were initially identified in baculoviruses as proteins that inhibit apoptosis of the host cells to allow time for viral replication. Cellular homologues containing at least one baculoviral IAP repeat (BIR) motif essential for their anti-apoptosis activity have been identified in yeasts and higher organisms and often act by binding and inhibiting processed caspases. The activity of these proteins can be modulated by the expression of proteins such as Smac/DIABLO and XAF-1 which displace or prevent the binding of caspases by IAPs. Recently, a mitochondrial serine protease termed Omi/HtrA2 has been found to bind IAPs. Similar to Smac, Omi possesses a conserved IAP-binding motif, but acts to cleave IAPs to irreversibly inactivate IAPs and promote apoptosis.