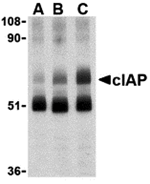
Apoptosis, or programmed cell death, is related to many diseases, such as cancer. Apoptosis is triggered by a variety of stimuli including members in the TNF family and can be prevented by the inhibitor of apoptosis (IAP) proteins. IAP proteins form a conserved gene family that binds to and inhibits cell death proteases. The two isoforms of c-IAP (c-IAP1 and c-IAP2) are structurally related to XIAP, containing 3 baculoviral IAP repeat (BIR) motifs that are essential and sufficient for the binding and inhibition of caspases-3, -7. The c-IAPs can associate with the death receptor TNF-R2, and mediate the ubiquitinization of TRAF2 following the binding of TNF-α by its receptor. Omi, a negative regulator of c-IAP, inhibits its activity by catalytically cleaving c-IAP. Another negative regulator, Smac/DIABLO, acts by enhancing the auto-ubiquitization activity of c-IAP.