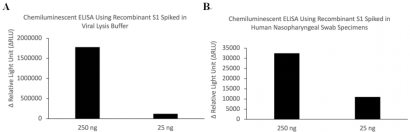
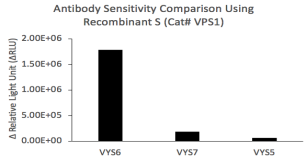
The single-stranded, plus-sense severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) genome encodes nonstructural replicase polyproteins as well as structural proteins such as spike (S), nucleocapsid (N), membrane (M) and envelop (E) proteins (Zhou et al., 2020). S protein is a transmembrane homotrimeric class I fusion glycoprotein; the S1 subunit binds to angiotensin-converting enzyme II (ACE2) and S2 subunit is involved in the fusion of viral and host cell membranes (Sternberg and Naujokat, 2020). The distal S1 subunit contains a receptor binding domain (RBD, residues Arg319-Phe541, GenBank accession No. YP_009724390.1), whose hinge-like conformational movement is required for ACE2 receptor binding and refolding of S2 for membrane fusion (Walls et al., 2020; Wrapp et al., 2020). Accumulating evidence from convalescent COVID-19 individuals supports that RBD is a highly immunogenic epitope targeted by neutralizing monoclonal antibodies through adaptive immune responses mediated by CD4+ T cells (Cao et al., 2020; Grifoni et al., 2020). Therefore, S and its RBD have been the targets for the design and development of vaccines to prevent SARS-CoV-2 infection and rechallenge (Sternberg and Naujokat, 2020).