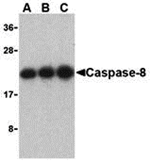
Caspases are a family of cysteine proteases that can be divided into the apoptotic and inflammatory caspase subfamilies. Unlike the apoptotic caspases, members of the inflammatory subfamily are generally not involved in cell death but are associated with the immune response to microbial pathogens. The apoptotic subfamily can be further divided into initiator caspases, which are activated in response to death signals, and executioner caspases, which are activated by the initiator caspases and are responsible for cleavage of cellular substrates that ultimately lead to cell death. Caspase-8 is an initiator caspase that was identified as a member of the Fas/APO-1 death-inducing signaling complex. The adaptor molecule FADD couples procaspase-8 to the Fas receptor death domain; subsequent oligomerization promotes procaspase-8 autoactivation. FLIP, a catalytically inactive caspase-8-like molecule inhibits these interactions and thus can inhibit apoptosis.