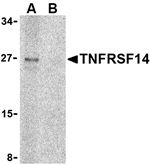
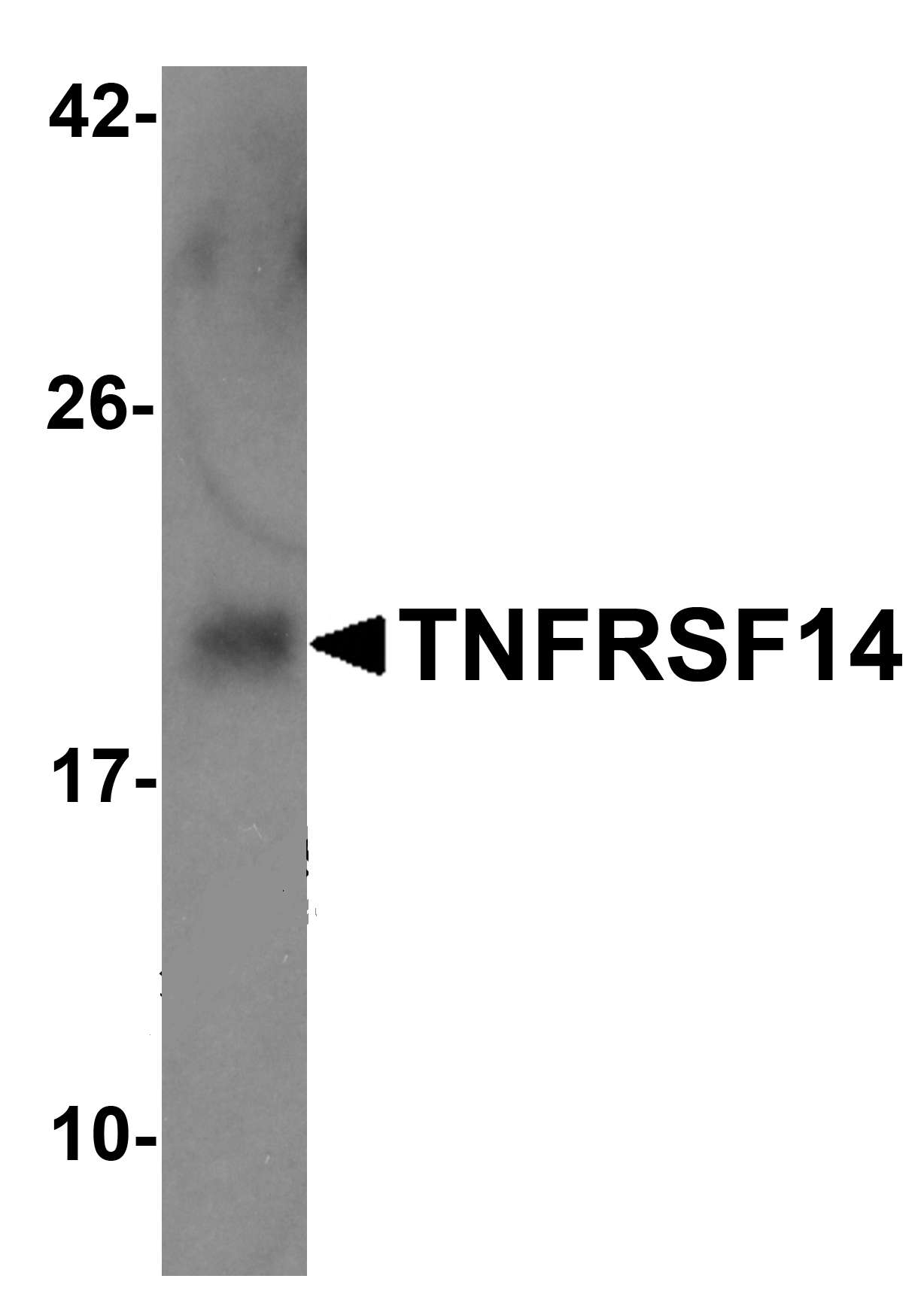
Tumor necrosis factor receptor (TNFR) superfamily members are defined by cysteine-rich domains in their extracellular regions that bind TNF-related ligands that share a common structural homology in their extracellular domain. TNFRSF14 was initially identified as the Herpesvirus entry mediator and upon binding to the herpes simplex virus (HSV) envelope glycoprotein D or either of its natural ligands LIGHT and lymphotoxin alpha (LT), activates the transcription factors NF-κB and AP-1. Activation of this signal transduction pathway in T cells stimulates T cell proliferation and cytokine production, leading to inflammation and enhanced CTL-mediated tumor immunity, suggesting that these proteins may be useful as potential targets for controlling cellular immune responses.