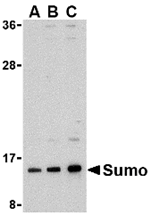
The sumo family of proteins is related both structurally and functionally to ubiquitin in that they are post-translationally attached to the e-amino group of a lysine residue of the substrate protein. This sumoylation plays a number of roles in DNA replication and repair, protein targeting to various subnuclear structures, and the regulation of numerous cellular processes including the inflammatory response in mammalian cells. Sumo was initially identified as a covalent modification of RanGAP1 in studies on nuclear import in mammalian cells. More recently, sumo has been shown to be involved in the regulation of transcription factors, possibly by enhancing their interactions with co-repressors. Sumo is also thought to play some role in the modulation of ubiquitin-mediated degradation of proteins by acting as an inhibitor. At least four different isoforms of sumo are known to exist; Sumo antibody will only recognize isoform 1.