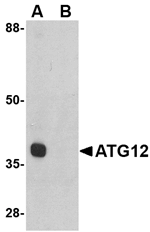
Autophagy, the process of bulk degradation of cellular proteins through an autophagosomic-lysosomal pathway is important for normal growth control and may be defective in tumor cells. It is involved in the preservation of cellular nutrients under starvation conditions as well as the normal turnover of cytosolic components. This process is negatively regulated by TOR (Target of rapamycin) through phosphorylation of autophagy protein APG1. ATG12, another member of the autophagy protein family, forms a conjugate with ATG5; this conjugate has a ubiquitin-protein ligase (E3)-like activity for protein lipidation in autophagy. This conjugate also associates with innate immune response proteins such as RIG-I and VISA (also known as IPS-1), inhibiting type I interferon production and permitting viral replication in host cells. ATG12 has also been shown to interact with ATG10 in human embryonic kidney cells in the presence of ATG7. At least two isoforms of ATG12 are known to exist.