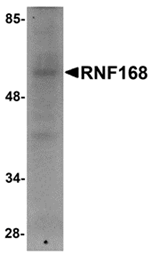
RNF168 was identified as a chromatin-associated RING finger protein that acts as a ubiquitin ligase both in vitro and in vivo. RNF168 targets histones H2A and H2AX, but not H2B, forming K63 polyubiquitin chains. Upon formation of DNA double strand breaks, RNF168 is recruited to the site of DNA damage where it co-localizes with gammaH2AX and 53BP1 in an RNF8-dependent manner. This localization of RNF168 increases the local concentration of ubiquinated proteins to the threshold required for retention of the proteins 53BP1 and BRCA1, facilitating the downstream signaling cascade. Thus, RNF168 defines a new pathway demonstrating a functional cooperation between E3 ligases in genome maintenance. At least three isoforms of RNF168 are known to exist.