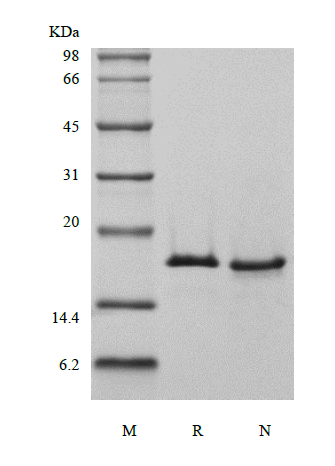
Ubiquitin-conjugating enzyme E2 D3 belongs to the ubiquitin-conjugating enzyme family and is encoded by the UBE2D3 gene in humans. The ubiquitin-conjugating enzymes, also known as E2 enzymes and more rarely as ubiquitin-carrier enzymes, take part in the second step in the ubiquitination reaction. In this reaction, E1 activates the ubiquitin by covalently attaching the molecule to its active site cysteine residue. The activated ubiquitin is then transferred to an E2 cysteine and then the E2 molecule binds E3 via a structurally conserved binding region. The ubiquitination reaction can modify proteins and regulate protein degradation. The UBE2D3 is a human homolog of the yeast UBC4/5 family and play many important regulatory roles in inflammation and cancer. It mediates the degradation of a myriad of short-lived regulatory proteins (such as p53 in the presence of E6/E6-AP or MDM2, c-Fos, IκBα, p105) and abnormal proteins and has 88% and 89% sequence identity with UbcH5a and UbcH5b respectively.