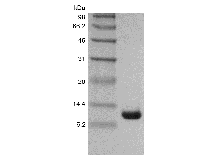
Cholera toxin (also known as choleragen and sometimes abbreviated to CTX, Ctx or CT) is protein complex secreted by the bacterium Vibrio cholerae. CTX is responsible for the massive, watery diarrhea characteristic of cholera infection. The cholera toxin is an oligomeric complex made up of six protein subunits: a single copy of the A subunit (part A, enzymatic), and five copies of the B subunit (part B, receptor binding), denoted as AB5. Subunit B binds while subunit A activates the G protein which activates adenylate cyclase. The five B subunits - each weighing 11 kDa, form a five-membered ring. The A subunit which is 28 kDa, has two important segments. The A1 portion of the chain (CTA1) is a globular enzyme payload that ADP-ribosylates G proteins, while the A2 chain (CTA2) forms an extended alpha helix which sits snugly in the central pore of the B subunit ring. This structure is similar in shape, mechanism, and sequence to the heat-labile enterotoxin secreted by some strains of the Escherichia coli bacterium.