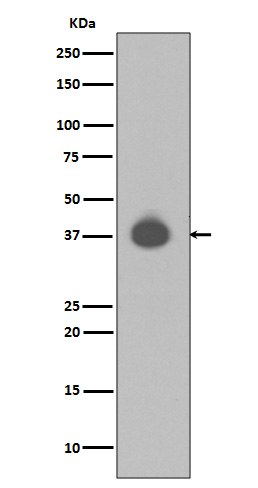
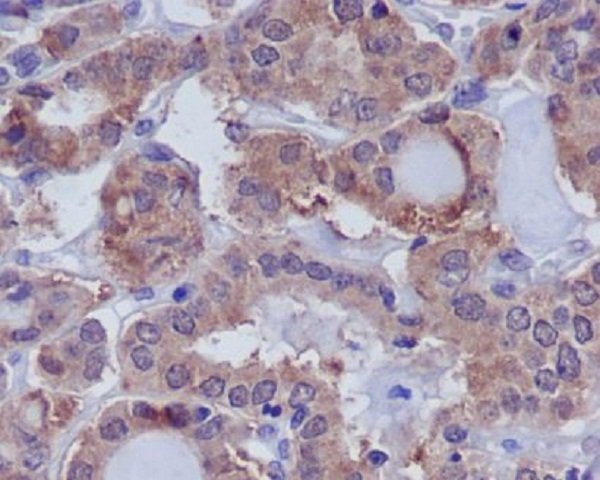
MAP kinases are inactivated by dual-specificity protein phosphatases (DUSP) that differ in their substrate specificity, tissue distribution, inducibility by extracellular stimuli and cellular localization. DUSPs, also known as MAPK phosphatases (MKP), specifically dephosphorylate both threonine and tyrosine residues in MAPK P-loops and have been shown to play important roles in regulating the function of the MAPK family. At least 13 members of the family (DUSP1-10, DUSp14, DUSP16, and DUSP22) display unique substrate specificities for various MAP kinases.