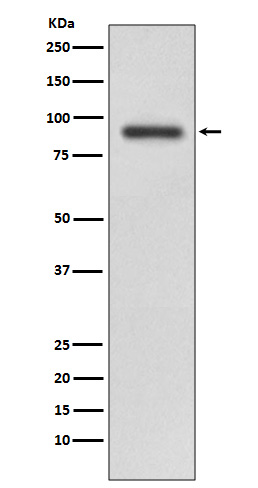
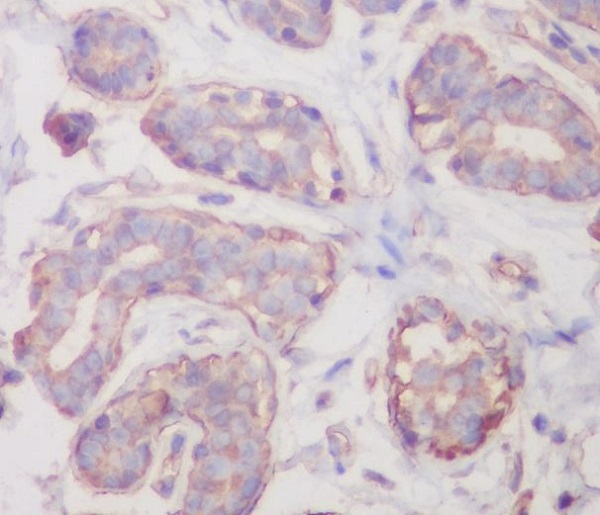
RalBP1 interacts with RalA and the endocytosis protein REPS2 (POB1) through its carboxy-terminal Ral binding domain. RalBP1 has an intrinsic GTPase activating function and interacts with Cdc42 through its centrally located Rho-GAP domain. A protein complex containing RalBP1/POB1/RalA regulates endocytosis of membrane receptors. RalBP1 also functions as a non-ABC transporter that catalyzes the ATP-dependent transport of numerous xenobiotics, including glutathione conjugates and some chemotherapeutic agents.