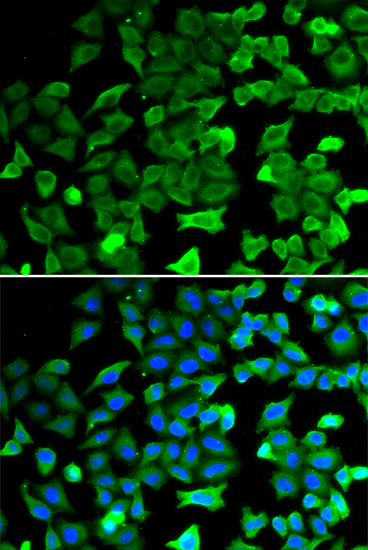
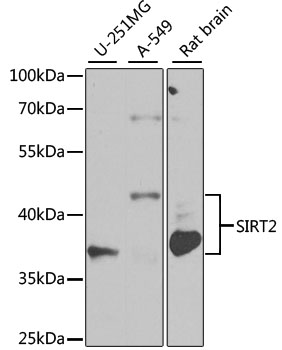
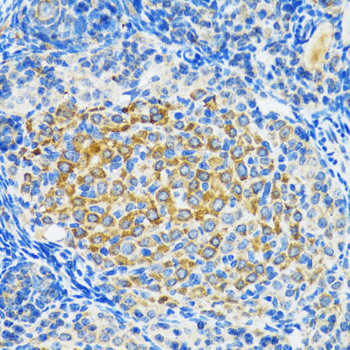
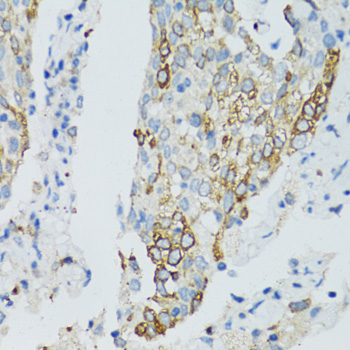
Sirtuins are members of the NAD-dependent histone deacetylase family of proteins that participate in a variety of cellular functions, including histone deacetylation, gene silencing, chromosomal stability, and aging. SIRT2, a human homolog of the yeast SIR2 (silent information regulator-2), functions as transcriptional silencing mediator at mating-type loci, telomeres and ribosomal gene clusters. SIRT2 expression increases dramatically during mitosis and is multiply phosphorylated at the G(2)/M transition of the cell cycle. SIRT2 is part of a phosphorylation cascade where it is phosphorylated late in G(2), during M, and into the period of cytokinesis. Inhibition of SIRT2 is reported to rescue alpha-synuclein toxicity and modify inclusion morphology in a cellular model of Parkinson's disease (1-4).