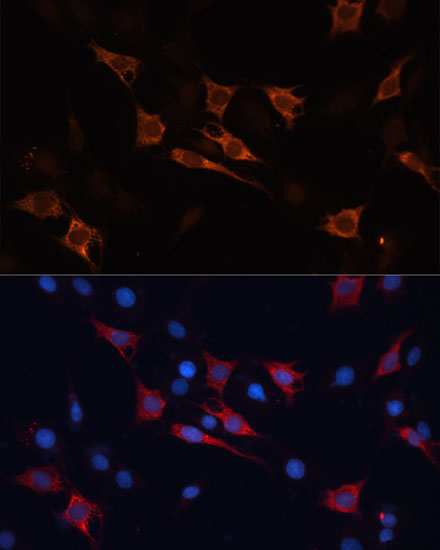
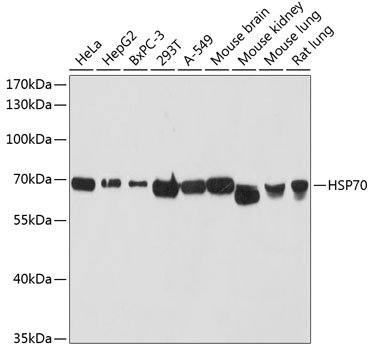
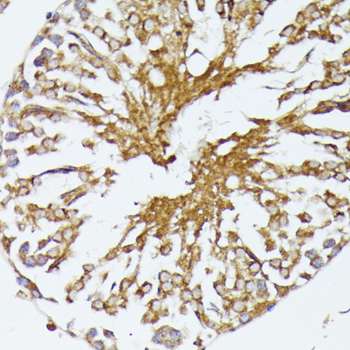
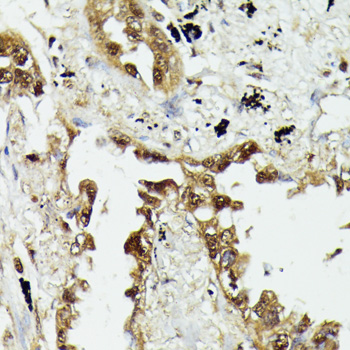
HSPA1A and HSP90 are molecular chaperones expressed constitutively under normal conditions to maintain protein homeostasis and are induced upon environmental stress (1). Both HSPA1A and HSP90 are able to interact with unfolded proteins to prevent irreversible aggregation and catalyze the refolding of their substrates in an ATP- and co-chaperone-dependent manner (1). HSPA1A has a broad range of substrates including newly synthesized and denatured proteins, while HSP90 tends to have a more limited subset of substrates, most of which are signaling molecules. HSPA1A and HSP90 often function collaboratively in a multi-chaperone system, which requires a minimal set of co-chaperones: HSP40, Hop, and p23 (2,3). The co-chaperones either regulate the intrinsic ATPase activity of the chaperones or recruit chaperones to specific substrates or subcellular compartments (1,4). When the ubiquitin ligase CHIP associates with the HSPA1A/HSP90 complex as a cofactor, the unfolded substrates are subjected to degradation by the proteasome (4). The biological functions of HSPA1A/HSP90 extend beyond their chaperone activity. They are essential for the maturation and inactivation of nuclear hormones and other signaling molecules (1,3). They also play a role in vesicle formation and protein trafficking (2).