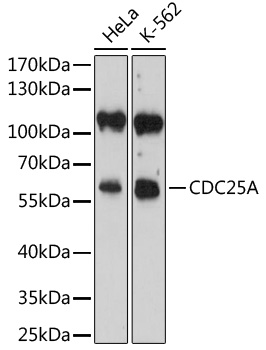
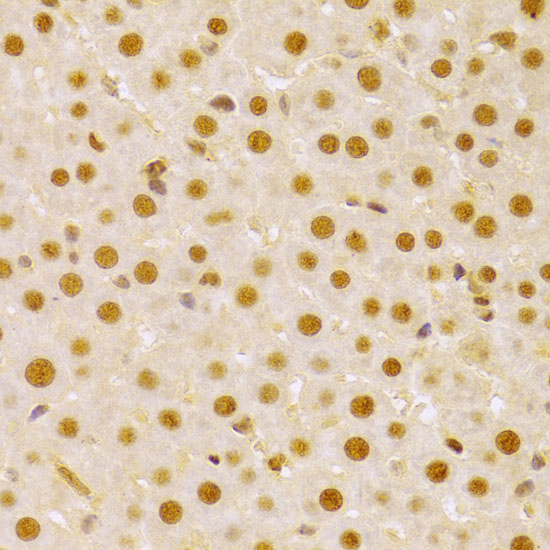
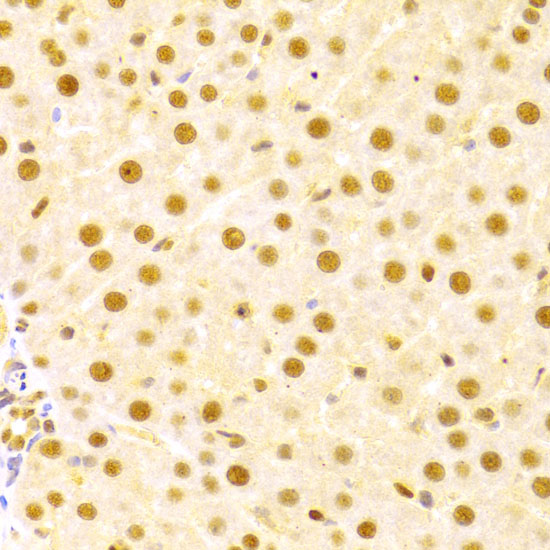
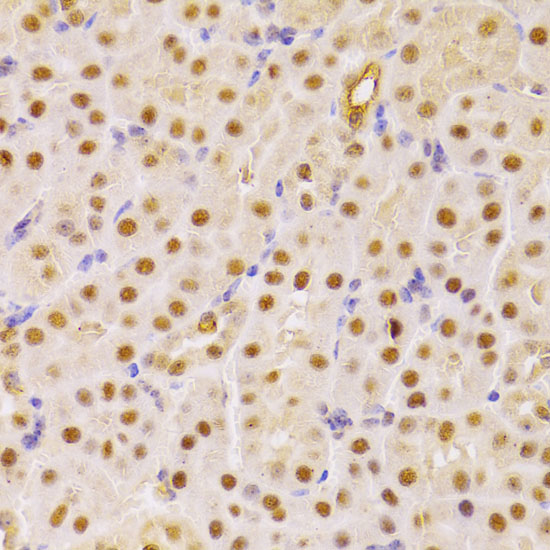
The cdc25 protein phosphatase family plays a critical role in activating cyclin-dependent kinases (CDKs) via dephosphorylation of conserved Thr14/Tyr15 inhibitory phosphorylation sites. While cdc25C is primarily responsible for activating CDK1 to overcome the G2/M checkpoint and allow mitotic entry, the primary substrate of cdc25A is CDK2, which, when active, allows progression through the G1/S and intra-S checkpoints (1). Abundance, subcellular localization and activity of cdc25A is tightly controlled by a variety of mechanisms, including phosphorylation, ubiquitination, and inhibitory binding to 14-3-3 proteins. During normal cell cycle progression, elevated c-Myc and E2F transcription factor levels lead to increased cdc25A expression (2). When conditions are favorable for DNA synthesis, cdc25A and CDK2 form an activation loop, wherein each activates the other enzyme (1). DNA damage, on the other hand, leads to multisite phosphorylation at inhibitory sites (Ser123, Ser177, Ser278, Ser292, and Thr506) by Chk1 and Chk2, which result in 14-3-3 binding and ubiquitin-mediated degradation (3,4).