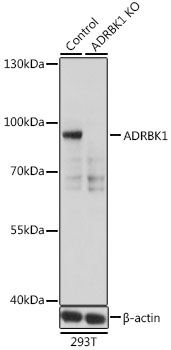
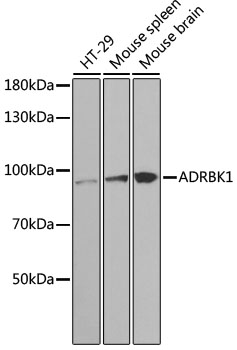
G-protein-coupled receptor kinase 2 (GRK2), also known as beta-adrenergic receptor kinase 1 (beta-ARK1), is a member of the GRK family, which phosphorylates the activated form of G-protein-coupled receptors (GPCRs) and initiates the desensitization process of GPCR (1). GRK2 kinase activity and cellular localization are tightly regulated by interactions with activated receptors, G-beta and G-gamma subunits, adaptor proteins, phospholipids, caveolin and calmodulin, as well as by phosphorylation (1). PKC phosphorylation enhances GRK2 activity by promoting its membrane localization and by abolishing the inhibitory association of calmodulin (2,3). PKA phosphorylates GRK2 at Ser685, which facilitates the association of GRK2 with a beta-adrenergic receptor (4). Erk inhibits GRK2 activity via phosphorylation at Ser670 (5). Src phosphorylates GRK2 at multiple tyrosine residues (Tyr13, 86 and 92), which activates GRK2 activity and promotes GRK2 degradation (6,7).