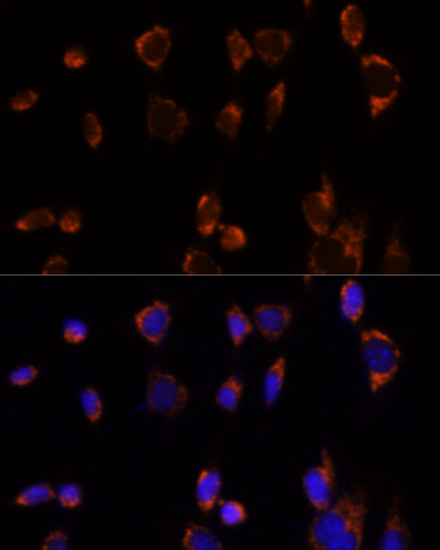
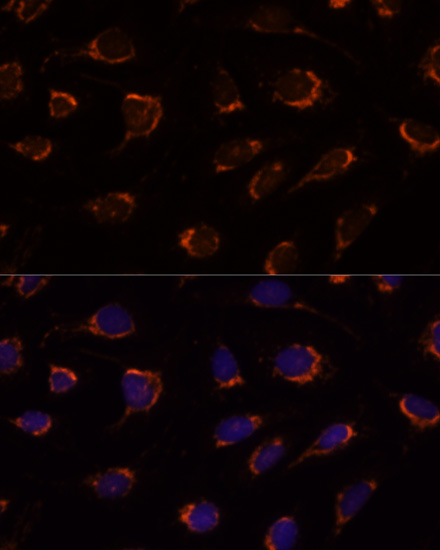
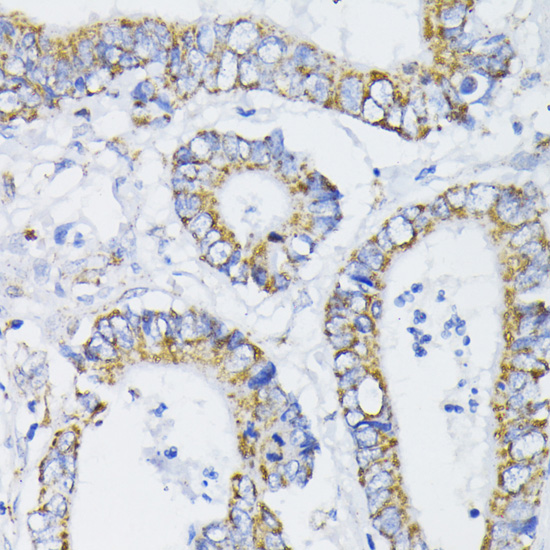
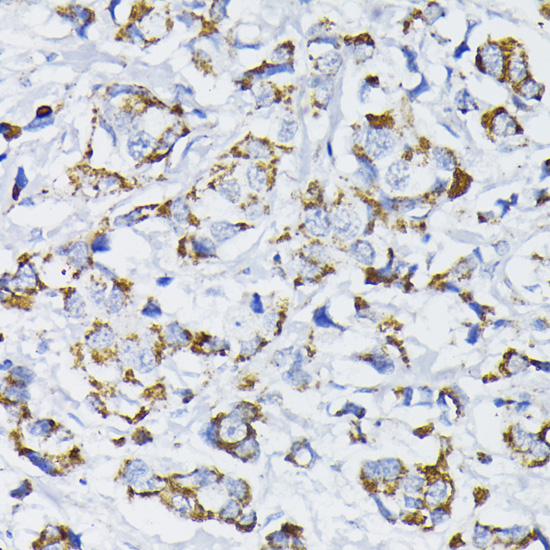
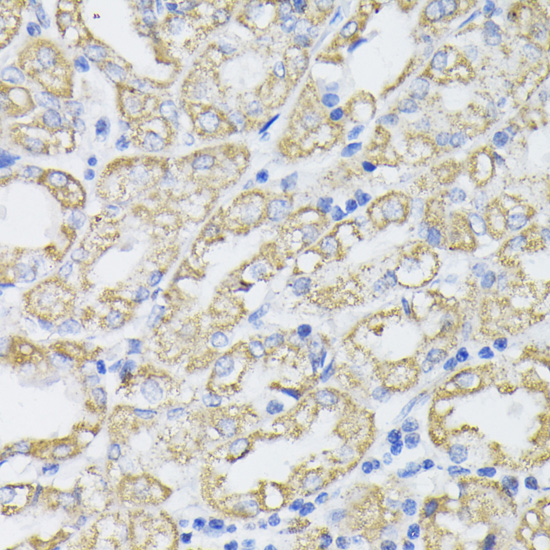
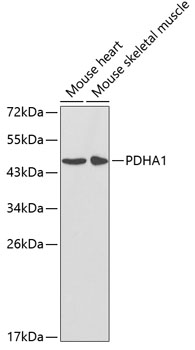
The pyruvate dehydrogenase complex catalyzes the conversion of pyruvate and CoA into acetyl-CoA and CO2 in the presence of NAD+. Acetyl-CoA then goes into the citric acid cycle where it reacts with oxaloacetate to form citrate. Acetyl-CoA is also used for fatty acid and cholesterol biosynthesis. The reaction of oxidative decarboxylation of pyruvate therefore serves as a critical link between glycolysis and the citric acid cycle and lipid metabolism. In mammalian cells, the pyruvate dehydrogenase complex is located in the mitochondrial matrix (1). This complex is comprised of three enzymes: pyruvate dehydrogenase (E1), dihydrolipoamide acetyltransferase (E2) and dihydrolipoamide dehydrogenase (E3). Pyruvate dehydrogenase (E1) consists of two subunits: α and β. This enzyme catalyzes the removal of CO2 from pyruvate. Mutations in the α subunits of pyruvate dehydrogenase (E1) lead to congenital defects that are usually associated with lactic acidosis, neurodegeneration and early death (2).