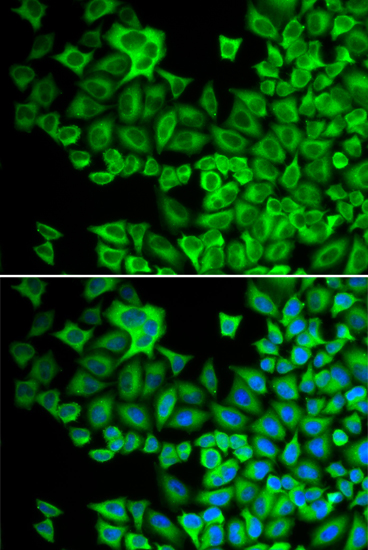
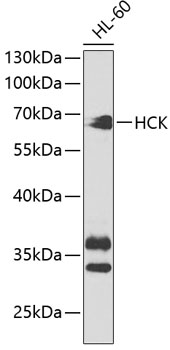
Hck (hemopoietic cell kinase) is a protein tyrosine kinase of the Src family prominently expressed in the lymphoid and myeloid lineages of hemopoiesis (1). It participates in transducing a variety of extracellular signals, which ultimately affect cellular processes including proliferation, differentiation and migration.The well-defined modular structure of Hck comprises a relatively divergent, NH2-terminal "unique" domain, which is subject to post-translational lipid modifications thereby targeting Hck to the plasma membrane. Src homology 3 (SH3) and 2 (SH2) domains, and a tyrosine kinase catalytic domain follow the "unique" domain. The catalytic activity of Hck is regulated, both positively and negatively, by tyrosine phosphorylation of highly conserved tyrosine (Y) residues. Phosphorylation of a single conserved Tyr499 residue in the COOH terminus of Hck by the protein kinase Csk renders Hck inactive as a result of an intramolecular interaction between the phosphorylated tyrosine (pY) residue and its own SH2 domain. Disruption of this interaction, either as a result of dephosphorylation, or substitution of the COOH-terminal regulatory Y residue with phenylalanine (F; e.g., HckY499F), or COOH-terminal truncation mutations as observed in the virally transduced v-Src oncoprotein, results in constitutive activation of Hck. In contrast to phosphorylation of the COOH-terminal regulatory tyrosine residue, autophosphorylation of a tyrosine residue (Tyr388) within the kinase domain of Hck acts to positively regulate its catalytic activity. Thus, activation of Hck requires both disruption of the COOH-terminal regulatory tyrosine-SH2 domain interaction and autophosphorylation of the regulatory tyrosine residue within the kinase domain ( 2, 3). The dysfunction or dysregulation of Hck may contribute to the pathogenesis of some human leukemias (4).