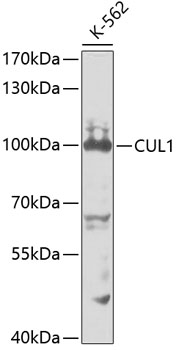
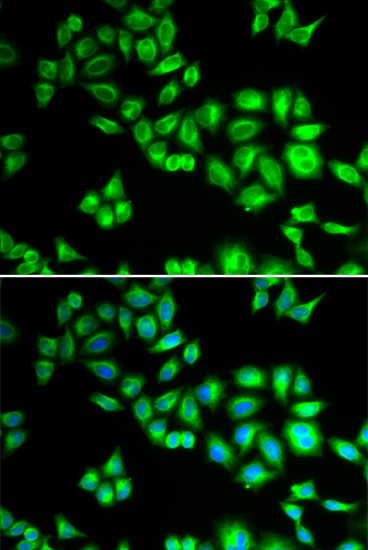
Ubiquitin can be covalently linked to many cellular proteins by the ubiquitination process, which targets proteins for degradation by the 26S proteasome. Three components are involved in the target protein-ubiquitin conjugation process. Ubiquitin is first activated by forming a thiolester complex with the activation component E1; the activated ubiquitin is subsequently transferred to the ubiquitin-carrier protein E2, then from E2 to ubiquitin ligase E3 for final delivery to the epsilon-NH2 of the target protein lysine residue (1-3). Combinatorial interactions of different E2 and E3 proteins result in substrate specificity (4). Recent data suggest that activated E2 associates transiently with E3, and that the dissociation is a critical step for ubiqitination (5). Cullin homolog 1 (CUL1), the mammalian homolog of Cdc53 from yeast, is a molecular scaffold of the SCF (Skp1/CUL1/F-box) E3 ubiquitin ligase protein complex. Thus, CUL1 and its family members function in ubiquitin dependent proteolysis (6). In particular, CUL1 has been shown to mediate ubiquitin dependent degradation of p21 Waf1/Cip1, cyclin D and IkappaB-alpha (7,8).