Product Detail
Product NameHemoglobin subunit gamma 1and2 Rabbit mAb
Clone No.JM84-10
Host SpeciesRecombinant Rabbit
Clonality Monoclonal
PurificationProA affinity purified
ApplicationsWB, ICC/IF, IHC
Species ReactivityHu
Immunogen Descrecombinant protein
ConjugateUnconjugated
Accession NoSwiss-Prot#:P69891
Uniprot
P69891
Gene ID
3047;
Calculated MW16 kDa
Formulation1*TBS (pH7.4), 1%BSA, 40%Glycerol. Preservative: 0.05% Sodium Azide.
StorageStore at -20˚C
Application Details
WB: 1:1,000-5,000
IHC: 1:50-1:200
ICC: 1:50-1:200
FC:150-1100
Western blot analysis of HBG1/2 on human placenta (1) and human brain (2) tissue lysates using anti-HBG1/2 antibody at 1/500 dilution.
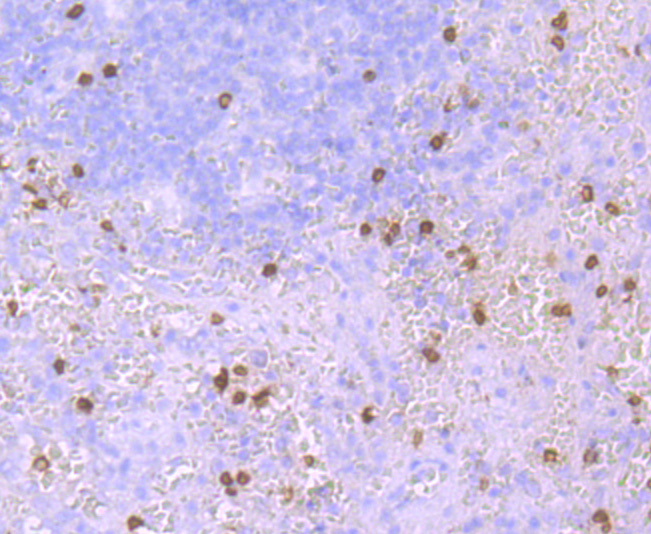
Immunohistochemical analysis of paraffin-embedded human tonsil tissue using anti-HBG1/2 antibody. Counter stained with hematoxylin.
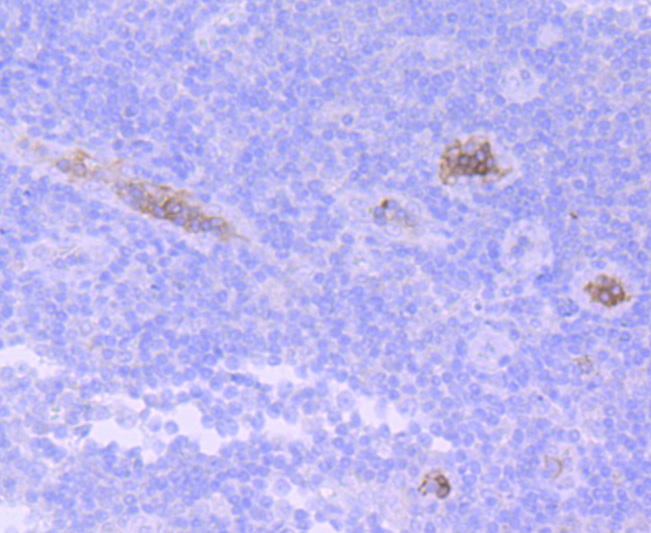
Immunohistochemical analysis of paraffin-embedded human spleen tissue using anti-HBG1/2 antibody. Counter stained with hematoxylin.
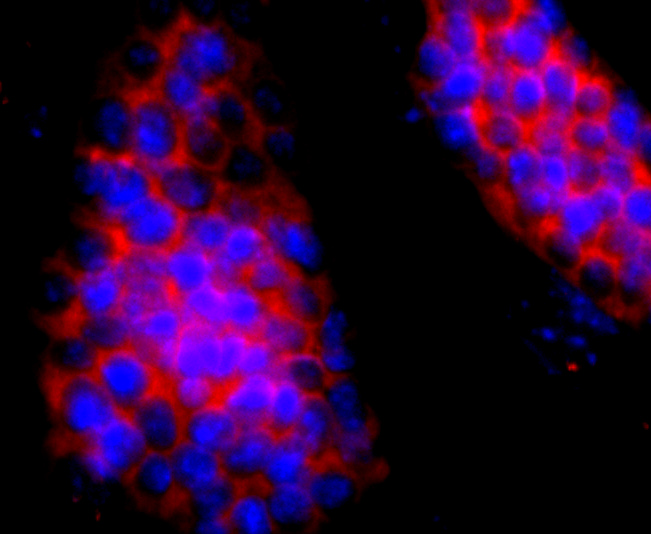
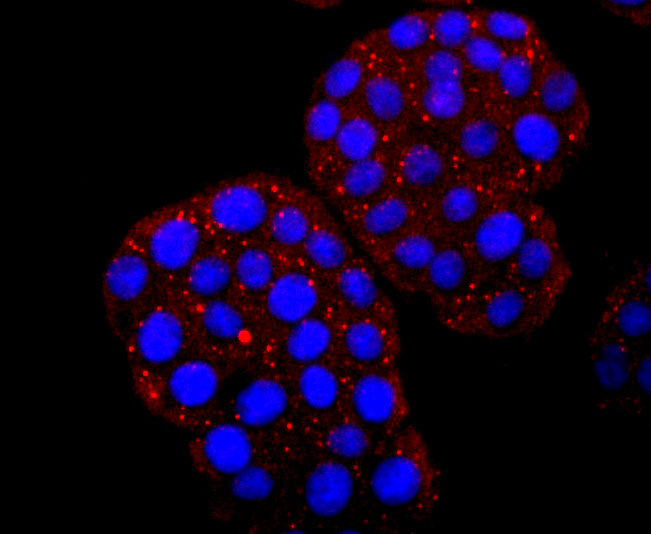
ICC staining HBG1/2 in D3 cells (red). The nuclear counter stain is DAPI (blue). Cells were fixed in paraformaldehyde, permeabilised with 0.25% Triton X100/PBS.
ICC staining HBG1/2 in MCF-7 cells (red). The nuclear counter stain is DAPI (blue). Cells were fixed in paraformaldehyde, permeabilised with 0.25% Triton X100/PBS.
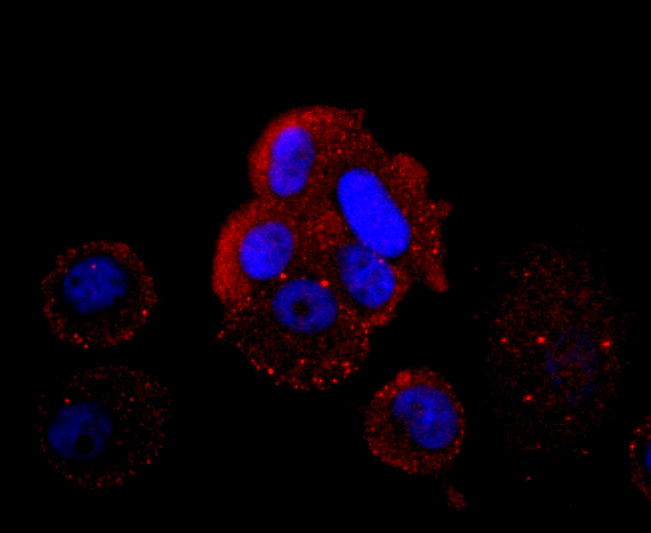
ICC staining HBG1/2 in PC-12 cells (red). The nuclear counter stain is DAPI (blue). Cells were fixed in paraformaldehyde, permeabilised with 0.25% Triton X100/PBS.
Hemoglobin (Hgb) is coupled to four iron-binding, methene-linked tetrapyrrole rings (heme). The α (16p13.3; 5-ζ-pseudoz-pseudo α2-pseudo α1-α2-α1-?1-3) and β (11p15.5) globin loci determine the basic hemoglobin structure. The globin portion of hemoglobin consists of two α chains and two β chains arranged in pairs forming a tetramer. Each of the four globin chains covalently associates with a heme group. The bonds between α and β chains are weaker than between similar globin chains, thereby forming a cleavage plane that is important for oxygen binding and release. High affinity for oxygen occurs upon relaxation of the α1-β2 cleavage plane. When the two α1-β2 interfaces are closely bound, hemoglobin has a low affinity for oxygen. Hb A, which contains two α chains plus two β chains, comprises 97% of total circulating hemoglobin. The remaining 3% of total circulating hemoglobin is comprised of Hb A-2, which consists of two α chains plus two δ chains, and fetal hemoglobin (Hb F), which consists of two α chains together with two γ chains.
If you have published an article using product 49459, please notify us so that we can cite your literature.



 Yes
Yes



