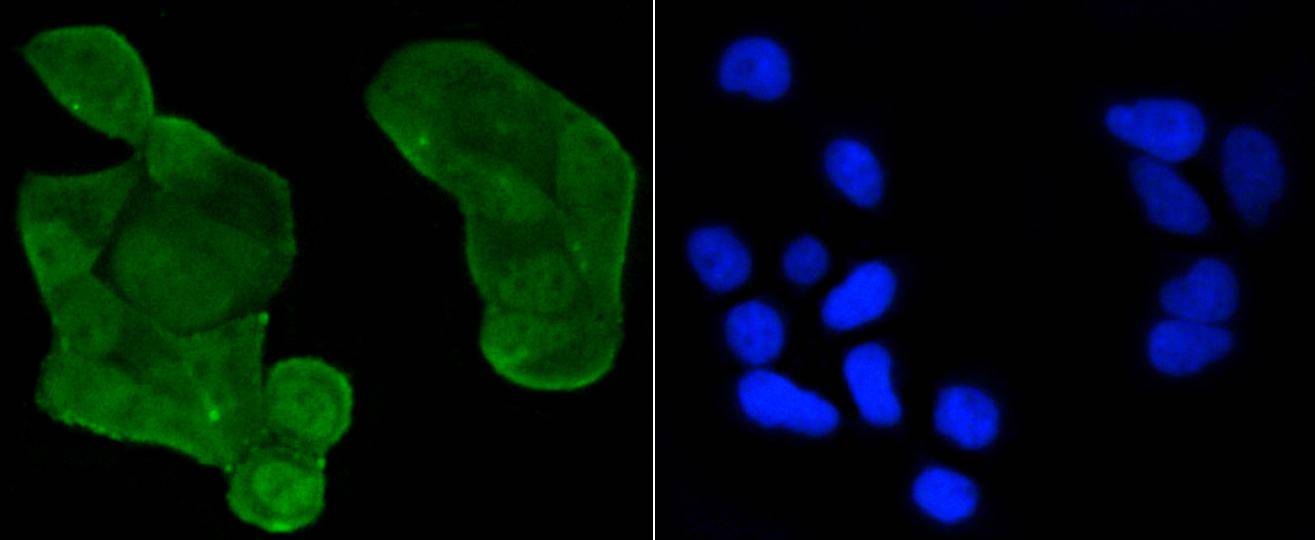
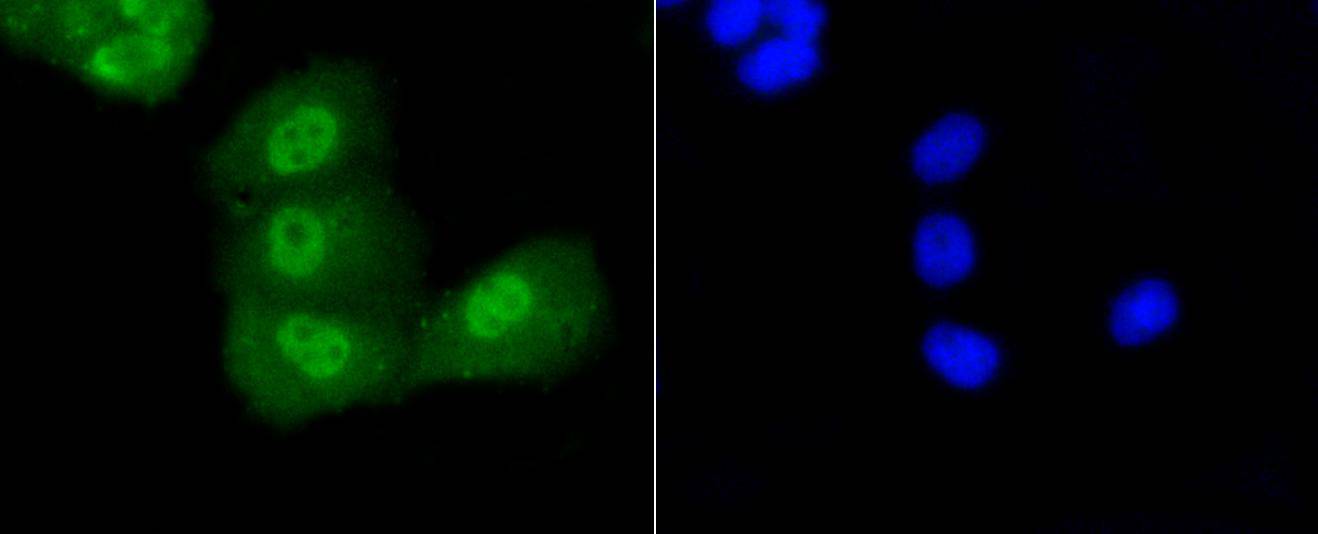
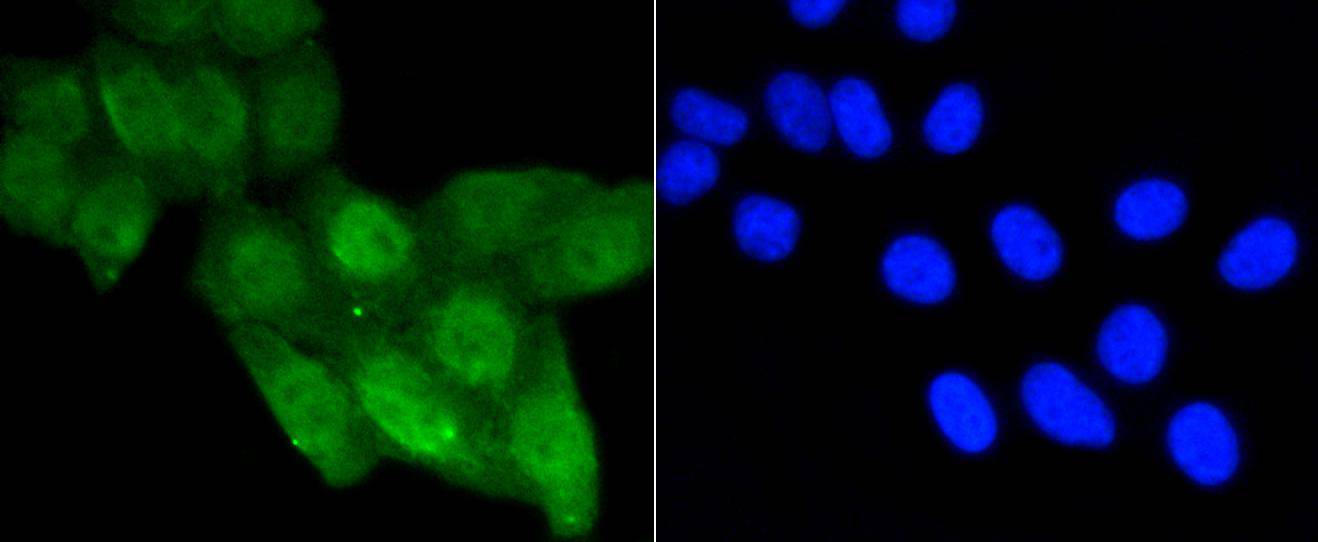
p53, a DNA-binding, oligomerization domain- and transcription activation domain-containing tumor suppressor, upregulates growth arrest and apoptosis-related genes in response to stress signals, thereby influencing programmed cell death, cell differentiation, and cell cycle control mechanisms. p53 localizes to the nucleus, yet can be chaperoned to the cytoplasm by the negative regulator, MDM2. MDM2 is an E3 ubiquitin ligase that is upregulated in the presence of active p53, where it poly-ubiquitinates p53 for proteasome targeting. p53 fluctuates between latent and active DNA-binding conformations and is differentially activated through posttranslational modifications, including phosphorylation and acetylation. Mutations in the DNA-binding domain (DBD) of p53, amino acids 110-286, can compromise energetically-favorable association with cis elements and are implicated in several human cancers.