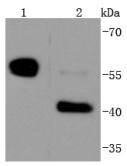
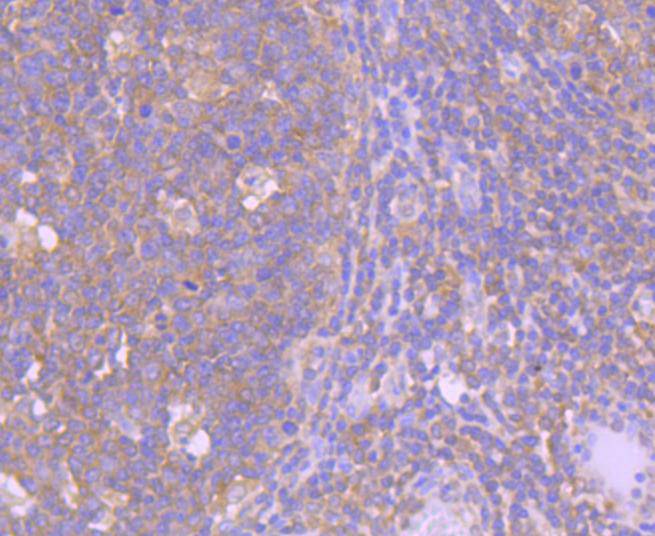
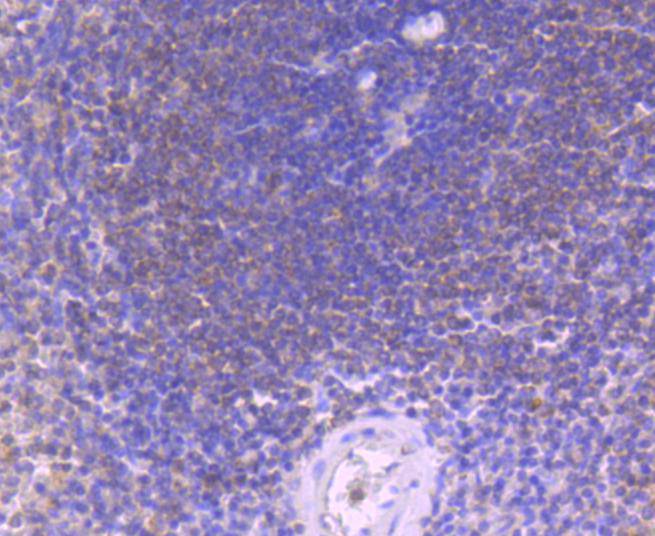
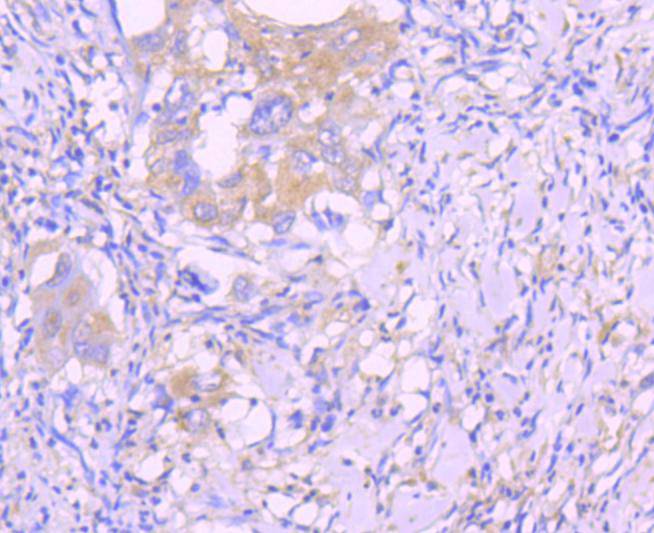
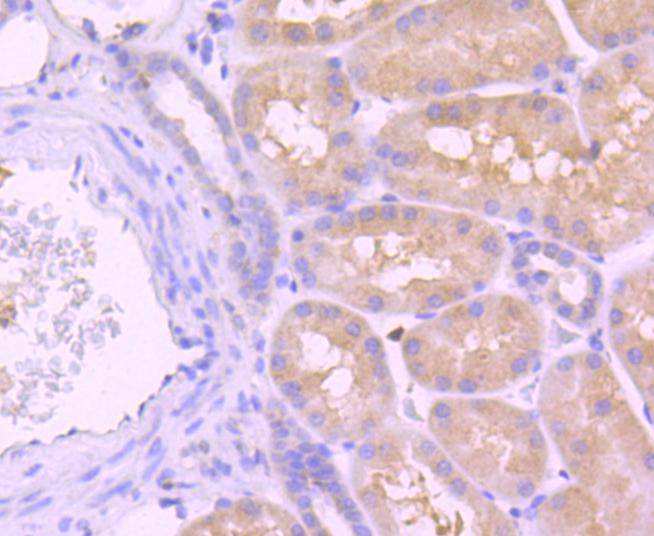
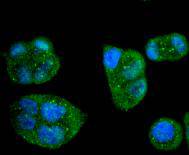
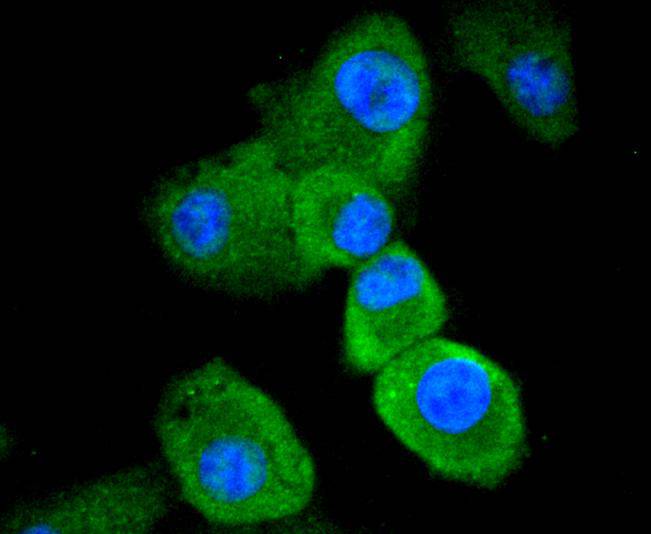
Initiator caspases, which include caspase-8, activate effector caspases by cleaving inactive forms of effector caspases. In the activation cascade responsible for apoptosis induced by TNFRSF1A and mediated by TNFRSF6/FAS, caspase-8 is the most upstream protease. Caspase-8 binds to adaptor molecule FADD, forming an aggregate referred to as death-inducing signaling complex (DISC), which activates caspase-8. The actived protein is released from the complex and further activates downstream apoptotic proteases. Caspase-8, which is a heterodimer consisting of two subunits (p18 and p10), is widely expressed, but is detected at highest levels in peripheral blood leukocytes (PBLs), thymus, liver and spleen. Defects in CASP8, the gene encoding for caspase-8, may cause CASP8D (caspase-8 deficiency disorder), which is characterized by splenomegaly and CD95-induced apoptosis of PBLs, may lead to immunodeficiency due to defects in T lymphocyte, NK cell and B lymphocyte activation.